1. Background
Type 2 diabetes is the most prevalent metabolic disease in the world, estimated to reach over 300 million cases by the year 2030 (1). As in other developing countries, the prevalence of diabetes is increasing in Iran, and it is estimated that more than 8% of Iranian people currently suffer from this disease (2).
Diabetes is a disorder with multiple etiologies, such as chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism that result from defects in insulin secretion, insulin action, or both, which characterizes type 2 diabetes (3). Diabetes and atherosclerosis are different conditions that are closely linked. Advanced cardiovascular disease is the most life-threatening situation for diabetic patients. Inflammation plays a major role in the processes of obesity, cardiovascular disease, type 2 diabetes, and other disorders (4). Inflammatory cytokines are important markers, and among them, IL-6 and TNF-α are often used to describe the degree of inflammation in patients with type 2 diabetes (5). Also, it has been indicated that diabetes increases oxidative stress and inflammation, and it decreases circulating adiponectin (6). Dietary components have been shown to affect the inflammatory process.
Hypoglycemic drugs can lead to unpleasant side effects, such as lactic acidosis, peripheral edema, severe hypoglycemia, and abdominal discomfort (7). For this reason, the search for new anti-diabetic agents has continued. It is well-documented that some natural antioxidants, such as polyphenols, have acute beneficial effects on endothelial function (8). Studies have shown that polyphenols may improve glucose and lipid disorders, and it is also established that they have anti-inflammatory effects (9).
Pomegranate (Punica granatum) is an ancient plant found in Iran. Its juice is polyphenol-rich, with high antioxidant properties (10). As demonstrated by in vivo and in vitro research, pomegranate and its juice have therapeutic hypoglycemic effects, including increased insulin sensitivity, inhibition of α-glucosidase, impact on the function of glucose transporter type 4, decreased total cholesterol (TC), and improved blood lipid profiles. In addition, anti-inflammatory action is exerted through modulation of the peroxisome proliferator-activated receptor pathway (11-14).
Concentrated pomegranate juice (CPJ), called “robe annar”, is used in Iran as a traditional additive. Several studies have suggested that reduced cholesterol biosynthesis in the liver might be due to the antioxidant activity of flavonoids. Some limited studies have investigated the effects of CPJ consumption on blood glucose and lipid concentrations in diabetic patients (15, 16). However, no previous study has assessed the effects of CPJ on inflammatory factors.
2. Objectives
As studies on the effect of CPJ on type 2 diabetes are limited, the present study was performed to assess the effects of CPJ on fasting blood sugar (FBS), lipid profiles, and inflammatory biomarkers, including high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), serum adiponectin, and total antioxidant capacity (TAC) in patients with type 2 diabetes.
3. Patients and Methods
3.1. Subjects
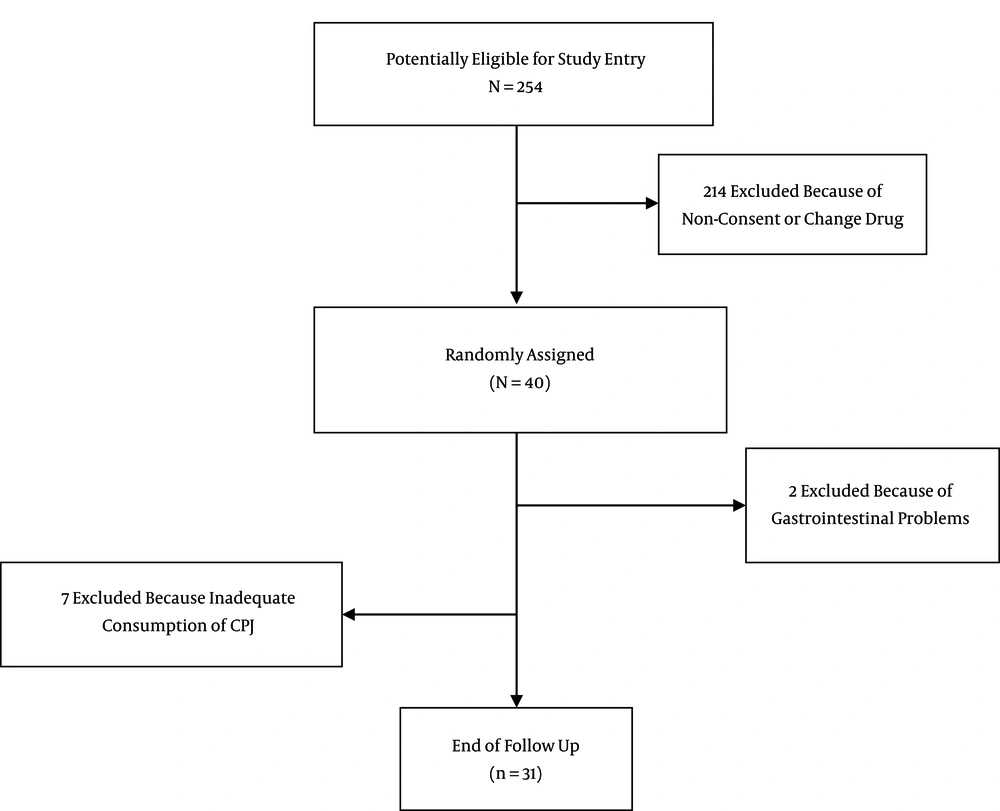
This quasi-experimental study was performed on 40 subjects aged 25 - 60 years with type 2 diabetes, who were recruited from the diabetes clinic at Golestan hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Participants had a mean systolic blood pressure of 124.25 ± 2.87 mmHg and a mean diastolic blood pressure of 83.02 ± 1.74 mmHg at baseline. The sample size, using NCSS software and a power of 90%, based on the results of an article on PJ with inflammation factors and significant IL-6, was calculated to be 31 subjects, assuming an estimated 30% dropout rate (13). An FBS of ˃ 126 mg/dL was the inclusion criterion. The exclusion criteria were as follows: the use of insulin, pregnancy or nursing, impaired renal function, active liver disease, and other chronic diseases. Subjects were also excluded if they consumed antioxidant or vitamin supplements. Oral hypoglycemic agents were taken by all of the patients except two, who controlled their diabetes with diet.
The CPJ was made from natural pomegranate juice with no added sugar, sweeteners, or preservatives; it was purchased from a local market in Mashhad, Iran. The total polyphenol concentration, determined spectrophotometrically, was 6.3 mg/100 g. The study protocol was approved by the Ethics Committee at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (NO: Rec.1392.148.B-9214). The study was registered at the Iranian registry of clinical trials under registration number IRCT2013091614680N1.
3.2. Anthropometric, Dietary, and Biochemical Measurements
Height and weight were measured with the participants in light clothing and barefoot. Height was measured to the nearest 0.1 cm and body weight was measured to the nearest 0.05 kg using a digital scale (Seca Clara 803, Hamburg, Germany). BMI was calculated as weight in kilograms divided by height in meters squared (m2). Waist circumference was measured at the minimal waist circumference between the last rib and the iliac crests (17). Blood pressure was measured on the left arm in a seated position after a 10-minute rest. All participants gave written informed consent.
The subjects were asked to consume 50 g of CPJ daily for 4 weeks. To ensure that the CPJ was taken regularly, all patients were closely followed with a weekly phone call. The subjects were also asked to carefully record their diet and physical activity, and to not change their usual diet, drug regimen, and physical activity during the study period. Dietary intake was assessed using 3-day food records at the beginning and end of the intervention period. The 3-day record was checked and completed by the dietician using color pictures of food. The total daily energy and nutrient consumption was estimated with nutrition 4 software (diet analysis module version 3.5.2, USA). The subjects’ mean physical activity level was assessed by using a standard physical activity questionnaire based on the metabolic equivalents of all activities during the previous week, including at work, during leisure time, and at rest or sleep (18).
3.3. Study Design
At the beginning of the experiment, venous blood samples (10 mL) were taken from all patients between 8:00 and 9:30 am after 12 - 14 hours of overnight fasting; this was repeated after the 4-week intervention period. The blood samples were centrifuged at × 1000 g for 10 minutes and stored at -70°C for further analysis. Fasting serum glucose was measured using glucose-oxidize (Pars Azmoon Co., Tehran, Iran). A commercially available enzyme-linked immunosorbent assay (ELISA) kit (Orgenium Laboratories Business Unit, Vantaa, Finland) was used for the measurements of TNF-α, IL-6, and adiponectin. labor diagnostic nord was used for hs-CRP according to the manufacture’s specifications. The inter-assay coefficients of variation were 8.6% for IL-6, 4% for TNF-α, and 12% for adiponectin. The corresponding intra-assays were 9.4%, 6%, and 10%, respectively. Sensitivity was 7 pg/mL for IL-6 and TNF-α, 10 ng/mL for hs-CRP, and 185 ng/mL for adiponectin.
3.4. Statistical Analyses
Statistical analyses were performed using SPSS for Windows (version 17; SPSS, Chicago, IL, USA). The normal distribution of variables was checked with the Kolmogorov–Smirnov test. The values are reported as mean ± SE. Paired t-test was used to compare the baseline with endpoint values. A P < 0.05 was considered statistically significant (Figure 1).
4. Results
Nine patients were excluded from the study due to inadequate consumption of CPJ or gastrointestinal problems, and 31 subjects (15 males, 16 females) completed the study. The age of the participants was 46 ± 8.3 years and the duration of diabetes was 4 - 10 years. The mean BMI of the participants was 29.53 ± 0.69 m2.
The comparison of anthropometric indices, metabolic factors, and inflammatory factors before and after of consumption of CPJ is presented in Table 1. BMI, weight, and waist circumference did not change after consumption of the CPJ. The intake of CPJ produced a significant increase in both TC and HDL-C from baseline. However, changes observed in serum triglyceride (TG), LDL-C, FBS, and blood pressure were not statistically significant.
| Variable | Week 0 | Week 4 | P Value |
|---|---|---|---|
| Fasting plasma glucose, mg/dL | 143.61 ± 7.22 | 140.77 ± 6.31 | 0.69 |
| TC, mg/dL | 161.09 ± 5.85 | 170.29 ± 5.84 | 0.03 |
| Triglyceride, mg/dL | 132.29 ± 17.37 | 145.19 ± 10.26 | 0.36 |
| HDL-C, mg/dL | 42.19 ± 1.27 | 43.90 ± 1.45 | 0.02 |
| LDL-C, mg/dL | 94.57 ± 4.41 | 98.81 ± 4.56 | 0.17 |
| TNF-α, pg/mL | 18.72 ± 0.95 | 17.66 ± 1.41 | 0.42 |
| Interleukin-6, pg/mL | 31.12 ± 3.12 | 23.40 ± 2.27 | 0.04 |
| hs-CRP, ng/mL | 2.37 ± 0.24 | 2.44 ± 0.23 | 0.74 |
| Adiponectin, ng/mL | 14.93 ± 0.28 | 14.07 ± 0.38 | 0.03 |
| Total antioxidant capacity, µm/L | 381.88 ± 20.54 | 1501 ± 146.90 | 0.001 |
| Systolic blood pressure, mmHg | 122.25 ± 3.37 | 119.32 ± 3.42 | 0.12 |
| Diastolic blood pressure, mmHg | 82.70 ± 2.08 | 80.70 ± 1.78 | 0.12 |
aValues expressed as mean ± SE.
bP values are from paired t-test.
Administration of CPJ for 4 weeks caused a significant reduction in serum IL-6, but TNF-α and hs-CRP remained unchanged. Compared to baseline, serum adiponectin levels were significantly decreased. The mean value of serum TAC was substantially increased (~ 75%), from 381.88 ± 114.4 at baseline to 1501 ± 817 after 4 weeks of CPJ consumption.
Energy, nutrient intake, and physical activity at baseline and after the 4-week intervention are presented in Table 2. The 3-day food records showed no significant changes in energy or nutrient intakes before and after the study. Physical activity levels also remained stable throughout the study.
| Variable | Week 0 | Week 4 | P Value |
|---|---|---|---|
| Energy, kcal/day | 2105 ± 121 | 1985 ± 75 | 0.30 |
| CHO, g/day | 319.34 ± 21.54 | 310.34 ± 15.91 | 0.66 |
| Protein, g/day | 76.37 ± 5.31 | 73.09 ± 3.08 | 0.52 |
| Fat, g/day | 60.92 ± 4.25 | 52.8 ± 2.77 | 0.08 |
| SFA, g/day | 15.03 ± 1.22 | 13.37 ± 0.75 | 0.24 |
| MUFA, g/day | 15.68 ± 1.16 | 14.44 ± 0.82 | 0.36 |
| PUFA, g/day | 21.66 ± 1.88 | 18.44 ± 1.37 | 0.12 |
| Sugar, g/day | 48.26 ± 9.86 | 68.74 ± 15.46 | 0.68 |
| Calcium, mg/day | 769 ± 51 | 680± 68 | 0.28 |
| Magnesium, mg/day | 222.35 ± 23.04 | 198.49 ± 13.77 | 0.34 |
| Zinc, mg/day | 6.82 ± 0.53 | 6.22 ± 0.34 | 0.20 |
| Cu, mg/day | 1.16 ± 0.13 | 1.09 ± 0.09 | 0.57 |
| Selenium, mg/day | 0.08 ± 0.01 | 0.07 ± 0.03 | 0.33 |
| Vitamin A, RE | 569 ± 79 | 641± 77 | 0.42 |
| Beta-carotene, ug/day | 216.30 ± 70.77 | 176.75 ± 47.64 | 0.59 |
| Vitamin E, mg/day | 4.19 ± 1.21 | 2.77 ± 0.64 | 0.31 |
| α-Tocopherol, mg/day | 7.20 ± 0.93 | 5.73 ± 0.55 | 0.12 |
| Vitamin C, mg/day | 75.72 ± 12.25 | 44.51 ± 8.76 | 0.06 |
| MET5, kcal/h.d | 2492 ± 140 | 2526 ± 141 | 0.56 |
Abbreviations: MET, Kcal/hour.day; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, Saturated fatty acid.
aValues expressed as mean ± SE.
bValues were derived from 3-day food records completed at baseline and the final week of the intervention.
cP values from paired t-test.
5. Discussion
We found that one advantage of CPJ intake on inflammatory factors in patients with type 2 diabetes was a significant decrease in plasma levels of IL-6. In addition, HDL-C levels were increased because of CPJ consumption. The daily consumption of 50 g of CPJ for 4 weeks had no effects on fasting serum glucose of diabetic patients. This finding is in agreement with most clinical trials that have examined the effects of PJ or CPJ on glycemic parameters in diabetic subjects (13, 16, 19, 20).
In a similar study by Rashidi et al. (15) neither FBS nor HbA1c changed after a 3-month administration of 45 g of CPJ per day in patients with type 2 diabetes. However, in another trial of diabetic patients, the consumption of 200 mL PJ for 6 weeks was associated with a significant reduction in FBS (21). We could not detect any significant correlations between CPJ and FBS, due to the different study duration or type of intervention in comparison to previous studies (21). It has been suggested that polyphenols, as the main component of pomegranate, might have hypoglycemic effects on different mechanisms, including increased glucose uptake by peripheral tissues, inhibition of glucose absorption in the gut, increased insulin release, or inhibition of gluconeogenesis (22). Thus, the overall results suggest that in diabetic patients, who usually avoid sugar-containing fruit, the intake of PJ or CPJ does not aggravate glycemic parameters.
This study indicated that 4 weeks of CPJ consumption increased TC and HDL-C levels in diabetic patients, but had no effects on serum TG. These results are in line with most previous studies showing that PJ did not alter serum TG levels in diabetic patients (13, 15, 23, 24). However, significant reductions of TC and LDL-C in diabetic patients with hyperlipidemia were reported after 6 weeks of 200 mL of PJ, and 8 weeks of 40 g CPJ, in diabetic patients with hyperlipidemia (20, 21). Regarding these differences, it seems that the hypocholesterolemic effects of PJ might be attributed to the higher baseline levels of TC and LDL-C of these patients, or to the duration of intervention in those studies.
In the present study, serum concentrations of TC increased significantly after administration of CPJ. Since this increase coincided with the significant increase in HDL-C levels, it can be concluded that the increased TC observed in our study was due to increased concentration of HDL-C. In line with this result, the HDL-raising effect of PJ has been reported in patients with non-alcoholic fatty liver disease (23). However, no changes in serum HDL-C were reported in other similar studies (4). It has been shown that polyphenols, via antioxidant activity, could protect HDL particles from oxidation and catabolism (25, 26).
Varying results have been reported regarding the effects of PJ or CPJ on blood pressure. In the present study, blood pressure did not show any significant reduction after intervention with CPJ, which is consistent with the results of a similar study that investigated the effect of 40 g of CPJ in type 2 diabetic patients with hyperlipidemia (16, 20). However, this finding is in contrast with the results of a clinical trial in which a significant reduction in blood pressure was reported after 2 weeks of PJ consumption in diabetic patients (27). Similarly, beneficial effects of PJ on blood pressure and serum angiotensin-converting enzyme ± ACE) activity in hypertensive patients have been reported in other studies (12, 24, 25, 28). This discrepancy can be attributed to different patient characteristics.
The concentration of circulatory cytokines, such as IL-6, CRP, and TNF-α, increased due to low-grade inflammation in diabetic patients (29). In the present study, a 33% reduction of serum IL-6 was observed following consumption of CPJ. This result is in agreement with a recent clinical trial that showed a 30% decline in serum IL-6 due to daily intake of 250 mL of PJ in diabetic subjects (30). Also, a significant constant decrease in IL-6 one month after drinking 240 ml/day of PJ was reported in adolescents with metabolic syndrome (31). Furthermore, a significant reduction of IL-6 levels was reported after one year of intake of PJ in hemodialysis patients (32). Another study showed a significant reduction in IL-6 after consumption of Punica granatum extract in patients undergoing periodontal treatment (33).
The mechanisms of the anti-inflammatory properties of PJ are not clear. However, it has been suggested that PJ inhibits the enzymes related to inflammation, such as peroxisome proliferator active receptors (PPARs), nuclear transcription factor kappa B (NF-κB), and NSAID activated gene-1 (NAG-1), which reduces pro-inflammatory cytokine secretion through the inhibition of MAP kinases (34).
In this study, no significant changes were observed in hs-CRP levels. Consistent with these findings, consumption of PJ for 4 weeks in patients with metabolic syndrome (13), and for 2 weeks in hypertensive patients, had no significant impact on hs-CRP (28). Another Iranian study of type 2 diabetes patients reported that PJ ± 240 cc for 8 weeks) did not decrease hs-CRP values compared to baseline (35). However, 12 weeks of PJ intake showed a significant reduction of serum hs-CRP in diabetic patients (36). These conflicting results could be due to the study duration of 4 weeks not being long enough to detect significant changes in this marker.
There was no significant effect of CPJ on serum TNF-α in the present study, which differs from the results of a study by Shema-Didi et al. that showed a significant reduction in TNF-α after one year of PJ intake by hemodialysis patients (32). PJ might suppress TNF-α-induced expression of cyclooxygenase, an enzyme that catalyzes the synthesis of prostaglandins, which are involved in pain and swelling during the inflammatory response (4). This discrepancy between the two studies could be attributed to differences between the pathological conditions of the participants and the duration of the interventions.
In the present study, a significant reduction of serum adiponectin was observed after 4 weeks of CPJ consumption. Since there has been no clinical trial investigating the effect of pomegranate on serum adiponectin, a comparison to our result is difficult.
Oxidative stress and lipid peroxidation are high in diabetic patients. Several phenol compounds, such as anthocyanins, punicalagins, ellagic acids, and hydrolysable tannins found in PJ (37), are well known for their effects on scavenging free radicals and preventing lipid oxidation (38). Our data revealed that ingestion of CPJ resulted in a significant rise in plasma TAC, nearly fourfold compared to baseline. Consistent daily intake of 100 g of fresh pomegranate fruit for 10 days among healthy subjects and daily intake of 100 mL of PJ for 2 weeks in adolescents, as well as 4 weeks of PJ administration in elderly subjects, led to significantly increased serum TAC (23, 39, 40). Furthermore, a significant increase in TAC and a decrease in serum malondialdehyde ± MDA), as a biomarker of oxidative stress, were also observed after 2 weeks of administration of 240 mL of PJ in healthy young men (41). The total polyphenol level in the CPJ in this study was 6.3 mg/100 g. As the dietary intake of our patients did not significantly change during the study period, it can be concluded that the increased plasma antioxidant capacity may be attributed to the polyphenols in PJ. As oxidative stress and lipid peroxidation in diabetic patients are high, antioxidant supplements such as polyphenols have been suggested to be useful. The antioxidant properties of PJ and its scavenging effects on free radicals have also been reported (41). We did not measure changes in plasma MDA or lipid peroxidation in the present study. However, previous studies have shown that daily intake of pomegranate polyphenols significantly decreased MDA and lipid peroxidation in patients with type 2 diabetes (39). These results indicate that CPJ administration in diabetic patients substantially improves their serum oxidative status.
To the best of our knowledge, this is the first study to investigate the effects of CPJ on inflammatory markers in diabetic patients, and which was well-controlled by frequent contact with the participants. Nevertheless, the one-group study design, without blinding of participants or a placebo group, was a limitation.
In conclusion, a 4-week intervention of 50 g of CPJ per day appeared to have favorable effects on certain markers of subclinical inflammation, and increased the plasma concentrations of antioxidants in diabetic patients. However, further research is necessary to better investigate the impact of CPJ on biomarkers of oxidative stress and inflammation.