1. Background
Gestational diabetes mellitus (GDM), glucose intolerance first diagnosed in pregnancy, has been a growing health problem among women in the last few decades (1). About 14% of pregnancies in the US and 4.8% in Iran were complicated with GDM (2, 3). Screening for GDM is recommended at weeks 24 - 28 weeks of gestation, according to the Iranian national screening program. Women at high risk are the exception to this guideline, and for these patients, the oral glucose tolerance test (OGTT) is performed at first prenatal visit. Risk factors for developing GDM include high maternal age, obesity, multiparity, previous history of GDM or complicated pregnancies, family history of type 2 diabetes, and Asian ethnicity (4-6).
Recently, hypovitaminosis D has also been suggested as a risk factor. Vitamin D (25-hydroxy vitamin D, or 25OHD) is a fat-soluble vitamin with skeletal and non-skeletal functions. It affects glucose metabolism through decreasing insulin resistance and improving insulin secretion (7). An inverse relationship between serum vitamin D levels and glucose metabolism in women with GDM has been reported in several observational studies (8-11). Hypovitaminosis D has also been associated with insulin resistance and reduced β cell function among individuals at risk of type 2 diabetes, including postpartum patients with a recent history of GDM (12).
Generally women with previous GDM are at higher risk of developing dysglycemia. A significant proportion of these patients develop isolated impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), and 5% - 14% of these cases convert to persistent type 2 diabetes even shortly after delivery (13, 14, 15).
A recent study showed that post-parturition vitamin D supplementation may prevent the progression of insulin resistance in patients with a recent history of GDM (16). However, to the best of our knowledge, the impact of prenatal supplementation on post-partum glucose metabolism has not yet been investigated.
2. Objectives
We therefore sought to explore the effect of vitamin D supplementation in women with GDM and hypovitaminosis D, both on post-partum glucose levels and insulin resistance, in a randomized clinical trial.
3. Patients and Methods
We conducted a balanced (1:1) randomized clinical trial at a single GDM clinic in Zanjan. Zanjan is a city in the northeast of Iran, 334 km from Tehran. The population of Zanjan is roughly 500,000. We founded a new and free GDM clinic for the participants of this project.
For the purposes of the present study, we selected from the referred pregnant women with GDM only those who met our eligibility criteria, which included: maternal age > 16 years, singleton pregnancy, and gestational age between 12 and 32 weeks (n = 117). The GDM cases were referred from primary health centers affiliated with Zanjan University of Medical Sciences, as well as private obstetric clinics throughout the city.
Diagnoses of GDM cases were made according to Carpenter-Coustan criteria or American diabetes association (ADA) criteria, usually at weeks 24 - 28 of gestation, but always in the first prenatal visit for high risk patients. Carpenter-Coustan criteria were defined as fasting, 1h, 2h, and 3h plasma glucose (PG) levels of 95, 180, 155, and 140 mg/dL after administration of 100 g OGTT, with or without a prior glucose challenge test (GCT, n = 99) (17). ADA criteria were defined as fasting, 1h, and 2h PG levels of 92, 180, and 153 mg/dL after one step 75 g OGTT (n = 18) (18). The ADA criteria were employed for GDM diagnosis after the national GDM screening protocol was changed.
Those women with known type 1 or 2 diabetes before pregnancy, a history of hypertension, or untreated thyroid disorders were not included. We excluded those women using assisted reproductive techniques (n = 1) or those with a history of high dose vitamin D consumption during the previous 3 months (n = 1).
The selected women were randomly assigned to receive either vitamin D in the intervention group (I) or no supplementation in the control group (C). The randomization was carried out by the statistician with a block size of four in a 2: 2 ratio. The random allocation papers were concealed in sequentially-numbered envelopes. After obtaining informed consent from the patients, baseline characteristics, and medical and obstetrical history were recorded in their medical profile by the investigators.
A blood sample was collected at the time of recruitment from all study subjects. Serum concentrations of 25OHD and calcium of those women in group I were measured immediately. However, the blood sample was stored at -80°C for women in group C, and was assayed for 25OHD at the end of trial. We excluded the subjects with sufficient basal serum vitamin D in group I initially, and in group C at the end of trial.
After excluding the patients with sufficient basal vitamin D, the remaining participants in group I were instructed to take 200,000 IU vitamin D3 for each of the first two days, and then 50,000 IU per week thereafter, up to 700,000 IU in total. Those at week 28 of gestation or later were asked to take 100,000 IU weekly. Compliance was self-reported by the patients, and was recorded in their medical profile. Patients were allowed to take supplements prescribed by their obstetrician, including multivitamins with calcium and 400 IU vitamin D3. All patients took the prescribed 700,000 IU vitamin D3 before delivery, and there were no cases in which vitamin D3 consumption was stopped.
After taking 400,000 IU vitamin D3, a random urinary Calcium/Creatinine (Ca/Cr) test was performed as a safety measure against vitamin D toxicity, which is the first sign of hypervitaminosis D (25OHD > 100 ng/mL) (19). Vitamin D supplementation was stopped if the urinary Ca/Cr (mg/dl/mg/dL) level was ≥ 1 with associated hypervitaminosis D.
Both groups received the routine prenatal care for GDM. Insulin was administered in the required cases and adjusted in weekly visits up to delivery.
The primary outcomes were maternal fasting plasma glucose (FPG), 2-h post 75 g glucose load plasma glucose (2-hPLG), fasting serum insulin, homeostasis model assessment of insulin resistance (HOMA-IR), HbA1C, and serum 25OHD at 6 - 12 weeks after delivery. The secondary outcomes were maternal, including pre-eclampsia, pre-term labor, type of delivery, and neonatal data including abortion, stillbirth, birth weight, hypoglycemia, congenital anomalies, macrosomia, and hyperbilirubinemia that required hospital admission and phototherapy. These data were elicited from hospital records.
Six to twelve weeks after delivery, and after 12 hours overnight fasting, a 75 g OGTT was performed. After glucose measurement, the sera and whole blood were kept at -80°C and 4°C, respectively, for testing of lipids, insulin, and HbA1C.
Serum 25OHD levels were determined by the ELISA (enzyme-linked immunosorbent assay) method (Immunodiagnostic Systems Ltd., Tyne & Wear, UK); the intra-assay CV were 5.3%, 5.6%, and 6.7%, at 15.6, 26.8, and 66 ng/mL, respectively, and the inter-assay CV were 4.6%, 6.4%, and 8.7%, at 16.1, 28.8, and 52.8 ng/dL, respectively. Insulin was measured by the ECL (electrochemiluminescence) method (Roche Diagnostic Co., Mannheim, Germany); the CV were 2.6%, 2.8%, and 2.5%, at 6.3, 20.9, and 747 µu/mL, respectively. Blood glucose measurement was carried out by the enzymatic (GOD - glucose oxidase) method (Pars Azmun Co., Tehran, Iran), and HbA1C measurement was performed with the enzymatic method (Sekisui Medical Co., Osaka, Japan).
Hypovitaminosis D was defined by serum 25OHD levels < 30 ng/mL (75 nmol/L). Vitamin D insufficiency and deficiency were defined as serum 25OHD levels of 20 - 30 ng/mL (50 - 75 nmol/L) and < 20 ng/mL (50 nmol/L), respectively (20). HOMA-IR was calculated with the Formula 1:

To calculate the study sample size, we assumed the frequency of dysglycemia after delivery in the control group to be 35% (21), the efficacy of intervention to be a 25% reduction of the frequency of dysglycemia, type I error to be equal to 0.05 for a study power of 80%, and a 10% drop out rate. The estimated required sample size was 110 in total. Dysglycemia was defined as the development of IFG, IGT, or type 2 diabetes in subjects as measured by the post-partum tests. IFG was defined by FPG levels of 100 to 125 mg/dL, IGT by 2-hPLG levels of 140 to 199 mg/dL, and type 2 diabetes by FPG levels ≥ 126 or 2-hPLG levels ≥ 200 mg/dL (18).
Statistical analyses were carried out using SPSS for Windows, version 11.5 (SPSS Inc., Chicago, USA). Data are presented as frequencies, means, and standard deviations. Quantitative data were analyzed with the Shapiro-Wilk test for normal distributions. The independent samples t-test was used for comparing the means of continuous variables between groups, and the Chi-square test was used to compare the categorical variables between study groups. Logistic regression analysis was performed to detect the independent risk factors of post-partum dysglycemia, including age, pre-pregnancy BMI, parity, history of type 2 diabetes in first degree relatives, history of previous GDM in the patient, and secondary serum 25OHD, as independent variables. P values less than 0.05 were considered statistically significant. This study was approved by the ethics committee of Zanjan University of Medical Sciences. This trial was registered at Iranian registry of clinical trials as IRCT2012101611144N1.
4. Results
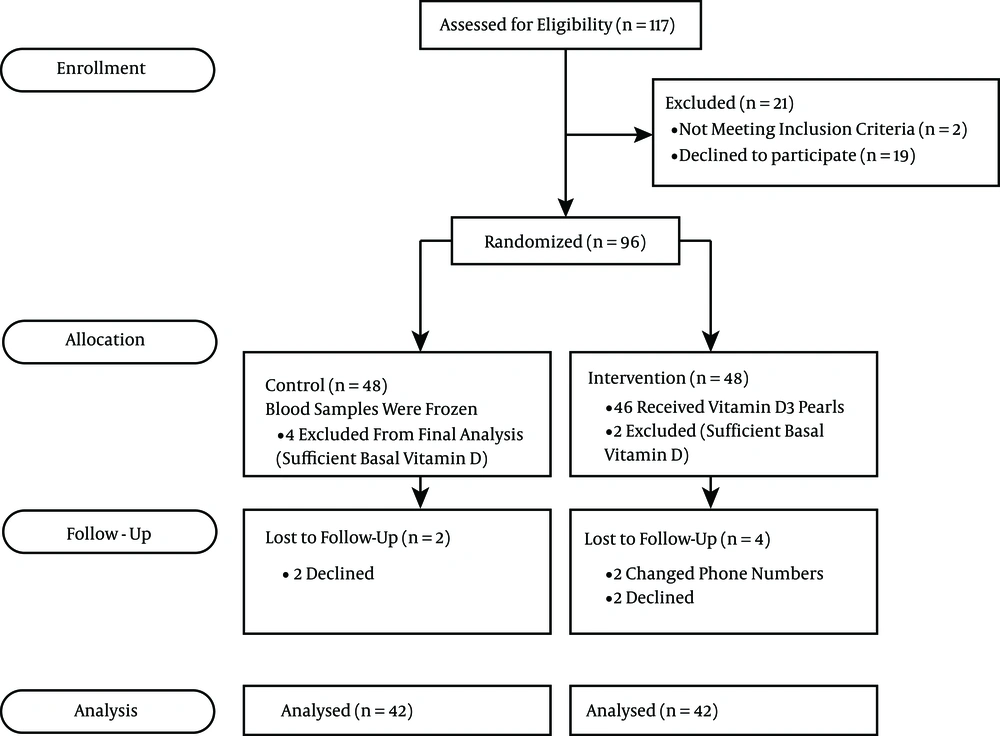
One hundred and seventeen pregnant women were recruited to the investigation between June, 2012 and May, 2014. Nineteen declined and two were excluded before randomization. Ninety six women with GDM at weeks 12 - 32 of gestation were randomly assigned to either the intervention (n = 48) or control group (n = 48). The study flow diagram is shown in Figure 1.
Baseline characteristics of the study groups are shown in Table 1. There was no significant difference between intervention and control groups in terms of age, pre-pregnancy BMI, gestational age at GDM diagnosis, and PG levels in OGTT.
| Variables | Intervention Group | Control Group | P Value |
|---|---|---|---|
| Number | 42 | 42 | |
| Age, mean (SD), y | 32.0 (5.5) | 32.4 (4.7) | 0.70 |
| Pre-pregnancy BMI, mean (SD), kg/m2 | 27.6 (3.9) | 27.6 (3.8) | 0.94 |
| Weight gain during pregnancy, mean (SD), kg | 8.9 (4.6) | 6.7 (4.8) | 0.04 |
| Planning for pregnancy, n (%) | 26 (61) | 22 (52) | 0.37 |
| Literacy, n (%) | |||
| Less than high school | 19 (45) | 16 (38) | 0.57 |
| High school | 8 (19) | 12 (28) | |
| University | 15 (36) | 14 (34) | |
| Gravidity, n (%) | |||
| 1 | 12 (28) | 10 (24) | 0.09 |
| ≥ 2 | 30 (72) | 32 (76) | |
| History, n (%) | |||
| GDM in patient | 6 (14) | 1 (2) | 0.10a |
| Type 2 diabetes in first degree relatives | 18 (42) | 14 (33) | 0.36 |
| Multivitamin use | 36 (85) | 36 (85) | 1.00 |
| GA at GDM diagnosis, mean (SD), week | 20.8 (7.6) | 21.3 (7.2) | 0.78 |
| Biochemistry, mean (SD) | |||
| 25 OHD, ng/mL | 14.6 (6.3) | 17.7 (6.1) | 0.04 |
| Serum Calcium, mg/dL | 8.9 (0.6) | 9.0 (0.5) | 0.95 |
| FPG, mg/dl | 97 (18) | 97 (14) | 0.87 |
| 1h OGTT | 196 (26) | 203 (29) | 0.29 |
| 2h OGTT | 170 (35) | 168 (31) | 0.86 |
| 3h OGTT | 109 (36) | 120 (36) | 0.17 |
| HbA1C, % | 5.5 (0.7) | 5.3 (0.5) | 0.13 |
| HbA1C, mmol/mol | 37 (7) | 34 (6) | 0.13 |
Abbreviations: SD, standard deviation; DM, diabetes mellitus; GA, gestational age; GDM, gestational diabetes mellitus; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test.
aFisher’s exact test P value.
The initial serum 25OHD in group I was slightly lower than in group C (14.6 ± 6.3 ng/mL vs. 17.7 ± 6.1 ng/mL, P = 0.04).
Insulin was prescribed for 38 (90%) women in group I with a mean dose of 22.3 ± 22 units/day at the end of pregnancy, compared with 35 (83%) women in group C with a mean dose of 16.1 ± 19 units/day. This difference was not statistically significant (P = 0.33, P = 0.17).
No cases of hypercalcemia were reported among participants. There was one subject in group I with urinary Ca/Cr ≥ 1 and with no associated hypervitaminosis D (36 ng/mL). The subject continued vitamin D supplementation as specified in the study protocol.
Maternal and neonatal outcomes were not significantly different between the groups (Table 2). Two cases of still birth were recorded: one occurred at week 25 of gestation in group C, and the second fetal loss was at week 39 of gestation in group I. The median follow up time in the study groups was 26 weeks, and occurred between the earliest eligible gestational age (12 weeks) at recruitment and the last post-partum OGTT.
| Variables | Intervention Group | Control Group | P Value |
|---|---|---|---|
| Number | 42 | 42 | |
| Primary outcomes, mean (SD) | |||
| 25 OHD, ng/mL | 32.4 (14.4) | 19.3 (9.6) | < 0.001 |
| FPG, mg/dL | 94 (16) | 89 (13) | 0.12 |
| 2-hPLG, mg/dL | 115 (48) | 110 (36) | 0.56 |
| Serum Insulin, µu/mL | 8.7 (4.4) | 8.8 (9.7) | 0.99 |
| HOMA-IR | 2.0 (1.3) | 1.8 (1.9) | 0.58 |
| HbA1C, % | 5.6 (0.5) | 5.5 (0.5) | 0.24 |
| HbA1C, mmol/mol | 38 (5) | 37 (5) | 0.24 |
| Secondary outcomes, n (%) | |||
| Cesarean section | 20 (43) | 24 (54) | 0.29 |
| Pre-term labor | 3 (6.5) | 5 (11) | 0.48a |
| Pre-eclampsia | 1 (2) | 3 (7) | 0.35a |
| Stillbirth | 1 (2) | 1 (2) | 1.00a |
| Hypoglycemia | 4 (8) | 5 (11) | 0.73a |
| Macrosomia | 2 (4) | 1 (2) | 1.00a |
| Hyperbilirubinemia | 4 (8) | 8 (18) | 0.18 |
| Congenital anomaly | 1 (2) | 1 (2) | 1.00a |
| Birth weight, mean (SD), grams | 3232 (458) | 3015 (615) | 0.07 |
| Cord blood 25OHD, mean (SD), ng/mL | 37.7 (5.3) | 15.0 (6.8) | < 0.0001 |
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance.
aFisher exact test P value.
At 6 - 12 weeks post parturition, the mean serum level of 25OHD in group I (32.4 ± 14.4 ng/mL) was significantly higher than that in group C (19.3 ± 9.6 ng/mL, P < 0.001) (Table 2). The high supplement dose dramatically raised the mean 25OHD in group I (+17.6 ± 14.4 ng/mL, P < 0.001), leading to a 20% deficiency rate in this group, compared to 58% in group C (P = 0.001). Mean fasting PG, 2-hPLG, and HbA1C did not differ significantly between groups (P = 0.12, P = 0.56, and P = 0.24, respectively). Among all patients, 13 (31%) developed post-partum dysglycemia in each group, including 5 and 8 cases of IFG, 5 and 6 cases of IGT, and 5 and 1 cases of type 2 diabetes in group I and C, respectively (P = 0.36, P = 0.74, and P = 0.09).
HOMA-IR in group I was 2.0 ± 1.3, compared with 1.8 ± 1.9 in group C. This difference was not significant (P = 0.58) (Table 2).
In the logistic regression analysis, pre-gestational BMI, previous history of GDM, and a history of type 2 diabetes in first degree relatives were the significant independent risk factors for post-partum dysglycemia (Table 3).
| Variable | OR (95% CI) | P |
|---|---|---|
| BMI | 1.28 (1.1-1.5) | 0.006 |
| GDM history in patient | 7.7 (1.1-53.4) | 0.039 |
| Type 2 DM in first degree relatives | 3.64 (1.1-12) | 0.034 |
| Gravidity | 0.91 (0.56-1.5) | 0.716 |
| Secondary serum 25OHD | 1.02 (0.98-1.06) | 0.337 |
5. Discussion
To our knowledge, this is the first study to evaluate the effects of vitamin D supplementation during pregnancy on glucose metabolism shortly after delivery. Our study demonstrates that high dose vitamin D supplementation during pregnancy in women with GDM and impairment of 25OHD concentration has no effect on post-partum plasma glucose and insulin resistance. However, supplementation caused an elevated 25OHD serum concentration (≥ 20 ng/mL) in 80% of women even two months after delivery. Fifty eight percent of group C remained vitamin D deficient, which indicates that the usual multivitamin that is prescribed for a majority of pregnant women (containing 400 IU vitamin D3) is not adequate to correct hypovitaminosis D. Therefore, pregnant women need a higher dose of vitamin D than that typically consumed.
Several observational studies indicate that serum 25OHD has an inverse relationship with insulin resistance and incidence of GDM (22, 23), and that vitamin D deficiency is associated with type 2 diabetes (24). In addition, animal studies have demonstrated that vitamin D deficiency causes insulin secretion defects (25). However, there is scarce information about the effect of vitamin D supplementation on glucose metabolism, especially during pregnancy and after delivery.
A recently published study by Asemi et al. (26) revealed a positive effect of co-supplementation with vitamin D and calcium on insulin resistance during pregnancy. In this study, 28 participants in the intervention group were assigned to receive a total dose of 100,000 IU vitamin D3 and daily 1,000 mg calcium, and were compared with 25 participants in a placebo group. The fasting plasma glucose, insulin level, and HOMA-IR after six weeks of intervention decreased significantly compared to the control group. In that study, GDM diagnosis was based on 75-g glucose OGTT (the new ADA criteria), whereas in our study, 84% of the recruited patients were diagnosed by 100-g glucose OGTT, which detects severe cases of GDM (27). Another difference is that in Asemi et al. study, patients requiring insulin therapy during pregnancy were excluded, whereas in our study, 90% of the patients in group I and 83% in group C required insulin for glycemic control. These facts indicate the higher severity of our cases, which might have affected the final results. Moreover, we examined our cases at much longer time periods after intervention. Other possible causes of the lack of effect of this intervention include the significantly higher weight gain in group I (8.9 kg) in comparison to the weight gain in the control group 6.7 kg (P = 0.04). This could potentially affect the post-partum OGTT results, although the cause of this significant weight gain is not clear. Although we cannot ignore the likely effect of calcium synergism on the positive result in the Asemi study, a study by de Boer et al. (28) found that 1000 mg elemental calcium plus 400 IU vitamin D daily, in middle age and elderly women for a median of 7 years, did not reduce the incidence of diabetes and insulin resistance. In contrast with our study, in which all patients were evaluated for basal vitamin D insufficiency or deficiency, the proportion of the patients with initial hypovitaminosis was not clarified in the Asemi study.
In another investigation by Yap et al. (29) on 179 women with GDM, plasma glucose level and HOMA-IR were compared in two groups receiving high dose (5000 IU daily) and low dose (400 IU daily) vitamin D supplementation. They found that high dose supplementation for a mean of 14 weeks during pregnancy was not beneficial to the patients’ plasma glucose level or insulin resistance during pregnancy, but that their babies benefitted from the supplementation with decreased neonatal hypovitaminosis D. The authors concluded that the commencement of vitamin D supplementation may have been too late to cause any observable effects on β-cell function, and they suggested that earlier supplementation, even before pregnancy, might be effective. In our study, over a median interval of 26 weeks between commencement of vitamin D supplements and OGTT measurements, high dose vitamin D led to maternal and neonatal 25OHD level increases, but not to the predicted effects on glucose level and insulin resistance. This negative result was likely caused by inadequate doses of vitamin D3, time limitations, or the lack of a causal relation between vitamin D and type 2 diabetes. As mentioned by Yap, this result may indicate that it is actually dysglycemia that leads to defects in liver hydroxylation of vitamin D.
Vitamin D supplementation has diverse effects on the non-pregnant population. A study by von Hurst et al. (30) reported that daily 4,000 IU vitamin D3 supplementation for 6 months (720,000 IU total) significantly decreased insulin resistance in South Asian women with vitamin D deficiency and insulin resistance. However, decreases in insulin resistance were not significant in the first 3 months, although resistance was reduced. The authors concluded that 6 months is an appropriate time interval for observing the presumed effects on insulin resistance. Similarly, our patients received high dose vitamin D3 (700,000 IU) with a median follow up time of 6.5 months in the intervention group. No effects on fasting plasma glucose and insulin resistance were observed in our study, and no trend in the reduction of insulin resistance was detected. Thus, the effectiveness of longer periods of supplementation with vitamin D is doubtful.
In another study of the non-pregnant population, and consistent with our results, Davidson and colleagues found that a mean supplement dose of 88,865 IU vitamin D per week, in 56 subjects with hypovitaminosis D and pre-diabetes, had no effect on insulin resistance and plasma glucose levels at 3, 6, 9, and 12 months after intervention, compared to 53 subjects in a placebo group (31). In this study, application of a proper dose and duration of vitamin D supplementation led to a rapid increase in 25OHD, up to near 70 ng/mL, which was maintained for the duration of the study. The only limitation of this study appears to be the small sample size of the pre-diabetes population. However, it is doubtful that any significant difference would have been found with a larger sample size (32).
As previously discussed, a limited number of studies have evaluated the effect of vitamin D on glucose metabolism and insulin resistance, especially during and after pregnancy. Consistent with our results, these studies have found that vitamin D is ineffective or has uncertain effects.
Several strengths of our study should be noted. The study was designed to detect any effects of vitamin D on insulin resistance by allowing for adequate time between the start of supplementation and the measurement of outcomes, and with the fewest number of patients lost to follow up. In addition, maternal and neonatal outcomes were assessed at delivery, and we administered a reasonable dose of vitamin D sufficient to achieve the necessary serum vitamin D levels in group I.
However, our study also had several shortcomings. The sample size of the study was not sufficient to identify effects on maternal and neonatal outcomes, and a very large sample size would be necessary to detect these low-incident clinical outcomes. Another shortcoming of the study is that none of the investigators or the patients were blinded to the intervention. We did not attempt to compare the daily calorie intake and physical activity between the two study groups. However, similar recommendations were presented for all participants to balance their caloric intake. We did not adjust the supplementation dose of vitamin D3 based on the patient’s weight in order to provide an appropriate volume of distribution of vitamin D in every patient, and the results would have been more reliable by calculating the proper dose for each individual. Regarding the predicted prevalence of 35%, the detected incidence of dysglycemia (31%) might have led to an underestimation of the impact of vitamin D in our study. However, it is unlikely that a greater sample size would change the direction of the hazard ratio, which was found to be 1.02 (range: 0.98 - 1.06). In spite of these limitations, this is currently the only study in the literature that evaluates the influence of vitamin D supplementation during pregnancy on postpartum dysglycemia.
In conclusion, prenatal vitamin D supplementation in patients with GDM and hypovitaminosis D safely and significantly increases maternal and neonatal serum 25OHD. This increase persists for several weeks after delivery, but does not affect fasting glucose and insulin levels, insulin resistance, or clinical outcomes.