1. Background
Diabetes mellitus (DM) is a widespread metabolic disorder associated with increased risk of cardiovascular disease and other complications (1-4). Pathophysiological mechanisms underlying type 2 (T2DM) and coronary artery outcomes include inflammation and oxidative stress (5). The latter arises from an imbalance between the amount of oxidants produced and the antioxidant defense system. Increased activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPX) result in increased blood glucose and insulin levels, oxidative damage by free radicals, and the formation of highly toxic products such as malondialdehyde (MDA) and lipid peroxidation products that can lead to insulin resistance (6). Some studies have shown that oxidative stress and insulin resistance are associated with higher levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, C-reactive protein (CRP), and other markers of systemic inflammation (7). Inhibiting the expression of inflammatory and oxidative stress biomarkers as well as hyperlipidemia can contribute to the control of diabetes and related complications (8).
Traditional Chinese medicines are increasingly used as an adjunct to conventional treatments for T2DM to improve diabetic symptoms and prevent complications. (4, 9-11). For example, JianYuTangKang (JYTK) is composed of ZhiMu (Rhizoma anemarrhenae), GuiJianYu (Euonymus alatus), and Ciwujia (Acanthopanax senticosus). JYTK extracts reduce postprandial blood glucose, lipid, and insulin concentrations in rats with diabetic and increase SOD activity at high doses (12, 13). Integrating traditional Chinese and western approaches (JYTK plus metformin) in patients with T2DM may not only help to improve glycemia and insulin sensitivity, but may also alter diabetes-related lipid equilibrium to a greater extent than metformin alone (14). JYTK has antioxidant and anti-inflammatory characteristics; however, these observations are limited to animal models and there have been no clinical trials carried out on patients with diabetes. In a pilot study at PLA General Hospital, JYTK was used to treat T2DM and its complications, and showed no adverse effects.
2. Objectives
The present clinical trial extended these findings and investigated the effects of JYTK combined with metformin on the levels of SOD, MDA, and GPX as well as the inflammatory factors TNF-α and IL-6 in patients with T2DM.
3. Patients and Methods
3.1. Tablet Preparation
The 500 mg JYTK tablets (science and technology center at PLA general hospital, Beijing, China) contained 129 mg A. senticosus, 105 mg E. alatus, and 266 mg R. anemarrhenae, as determined by high performance liquid chromatography (15-17). JYTK or placebo tablets (4.5 g) were administered three times daily. Placebo tablets identical in appearance to JYTK tablets were prepared at the academy of military medical sciences (Beijing, China). Toast powder was used as the placebo and as the vehicle in JYTK tablets.
3.2. Study Design and Participants
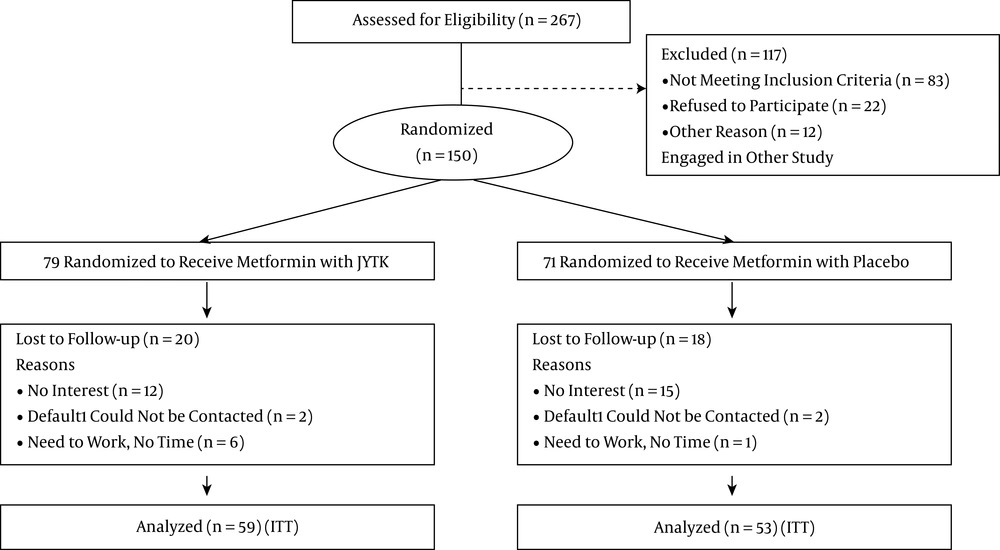
A total of 150 patients with T2DM, recently diagnosed (within the previous five years) according to the world health organization (WHO) criteria (18), were recruited from the PLA General Hospital outpatient service and Chaoyang district ShiLi BaoBeiLi and BaLiTun community service stations in Chaoyang district. The study used a randomized, placebo-controlled and double blind design. Of the 150 patients, 79 were assigned to the combined JYTK/metformin treatment group and 71 to the metformin-only group; 38 patients were lost to follow-up. Patients in both treatment groups were reimbursed for travel costs and time. The age range of the patients was 18 - 75 years. Patients with elevated glycosylated hemoglobin (> 6.5%), taking metformin for treatment and had a diabetes duration ≤ 5 years were included in the study. Exclusion criteria were patients taking other antihyperglycemic or antihyperlipidemic agents; receiving insulin therapy; with hematological diseases; with cardiac, renal, or hepatic diseases or tachycardia, depression, or myocardial infarction; the patients who consumed alcohol; had hypothyroidism or experienced vertigo or seizures; received treatment with estrogen, steroids, beta-blockers, or thiazide; and females who were pregnant, planning pregnancy, or breast-feeding. Patients were randomized using a computer-generated permuted block (1:1 allocation). Treatment doses were prepared by an independent operator, not otherwise involved in the trial, and concealed in opaque, sealed envelopes that were sequentially numbered.
3.3. Intervention
The treatment group received metformin 1.5 g /day and JYTK 4.5 g /day, and the control group received metformin 1.5 g /day and placebo 4.5 g /day. The investigators were blinded to the group assignment of patients using a coding system, with the codes known to an independent allocator and revealed only after completion of treatment and analyses. Patients were instructed to follow a diet recommended for newly diagnosed patients with T2DM and to walk 30 minutes a day at least three days a week during the trial. In the follow-up period, patients were phoned once a week to obtain their daily glucose levels and to supervise modifications to their lifestyle. It was reinforced at each follow-up visit (every two months). The number of patients lost to follow-up due to lack of interest, contact, or time to return for an interview is shown in Figure 1 using the flowchart model of the Consolidated standards of reporting randomized clinical trials 2010. Patients were informed about the benefits and possible risks of the study and were free to withdraw at any time during the trial for any reason. The complete list of inclusion and exclusion criteria was approved by the PLA general hospital ethics committee (study identifier: NCT00102589). Patients provided written, informed consent prior to enrollment.
3.4. Outcomes
Prior to intervention (baseline, week 0), at weeks 8 and 19, and at the end of the trial (26 weeks), overnight fasting venous blood samples (8 mL) were obtained from each patient between 07:00 and 09:00 hours. Serum was separated by centrifugation at 2500 rpm for 10 minutes and immediately stored at -80°C. MDA, SOD, and GPX levels were determined using commercially available kits (Nanjing Jiancheng bio-company, Nanjing, China). Serum TNF-α and IL-6 concentrations were determined using specific human sandwich enzyme-linked immunosorbent assay kits from ShanHai PuLinSiDun biological technology (Shanghai, China). Body weight, body mass index (BMI), waist and hip circumferences, blood pressure, and adverse effects were noted in medical records during the visits.
3.5. Statistical Analysis
An intent-to-treat analysis was carried out for all subjects and results are expressed as mean ± SD. Changes in biological parameters were examined by analysis of variance and the χ2 test using GraphPad Prism software (GraphPad, La Jolla, CA, USA). The Dunnett test for multiple comparisons was used to analyze data from the same group before and after treatment. Analysis of covariance (ANCOVA) was used to identify differences between the two groups after intervention, with results adjusted according to baseline measurements and covariates. Tests of treatment effects were carried out at a two-sided significance level of 0.05, and 95% confidence intervals for differences between baseline and week 26 within-group changes were determined and considered as statistically significant for values other than zero.
4. Results
4.1. Characteristics of the Study Population Following Treatment
All 112 subjects 59 in the JYTK + metformin group and 52 in the metformin only group completed the 26-week clinical trial; 38 were lost to follow-up due to lack of interest, contact, or time for a follow-up interview. All subjects who completed the study had acceptable compliance with the intervention, as determined by tablet counts, and did not report any adverse effects or symptoms associated with JYTK supplementation. There were no significant differences between groups in terms of demographic characteristics, fasting plasma glucose, urinary albumin-to-creatinine ratio, or complete blood count (P > 0.05) (Table 1). BMI was unchanged after 26 weeks of treatment (26.6 ± 6.9 to 26.7 ± 2.0 kg/m2 in the control group; 28.6 ± 4.8 to 29.7 ± 2.4 kg/m2 in the treatment group).
| Characteristics | Control Group Metformin+ Placebo (n = 53) | Treatment Group Metformin+ JYTK (n = 59)c |
|---|---|---|
| Age, y | 56.3 ± 11.1 | 54.9 ± 10.3 |
| Female | 59 (57.6) | 53 (51.0) |
| BMI, kg/m2 | 26.6 ± 6.9 | 28.6 ± 4.8 |
| FPG, mM/L | 8.1 ± 2.5 | 8.4 ± 1.9 |
| HbA1c, % | 7.2 ± 1.0 | 7.3 ± 1.1 |
| TC, mM/L | 5.1 ± 1.0 | 4.9 ± 0.9 |
| TG, mM/L | 1.7 ± 1.1 | 1.8 ± 1.0 |
| HDL-C, mM/L | 1.4 ± 0.4 | 1.3 ± 0.4 |
| LDL-C, mM/L | 3.0 ± 0.8 | 2.9 ± 0.8 |
| Hb, g/L | 147.8 ± 17.4 | 140.7 ± 14.5 |
| WBC, 109/L | 6.5 ± 1.7 | 6.4 ± 1.5 |
| NEU, % | 0.6 ± 0.08 | 0.6 ± 0.05 |
| ESR, mm/h | 10.4 ± 6.8 | 11.0 ± 7.2 |
| Hb, g/L | 147.8 ± 17.4 | 140.7 ± 14.5 |
| Cr, mM/L | 68.6 ± 18.9 | 64.6 ± 17.8 |
| UA, mM/L | 306.2 ± 91.2 | 299.9 ± 90.7 |
| ACR | 10.2 ± 6.8 | 9.5 ± 5.7 |
4.2. Combined JYTK/Metformin Treatment Decreases Oxidative Stress and Inflammation
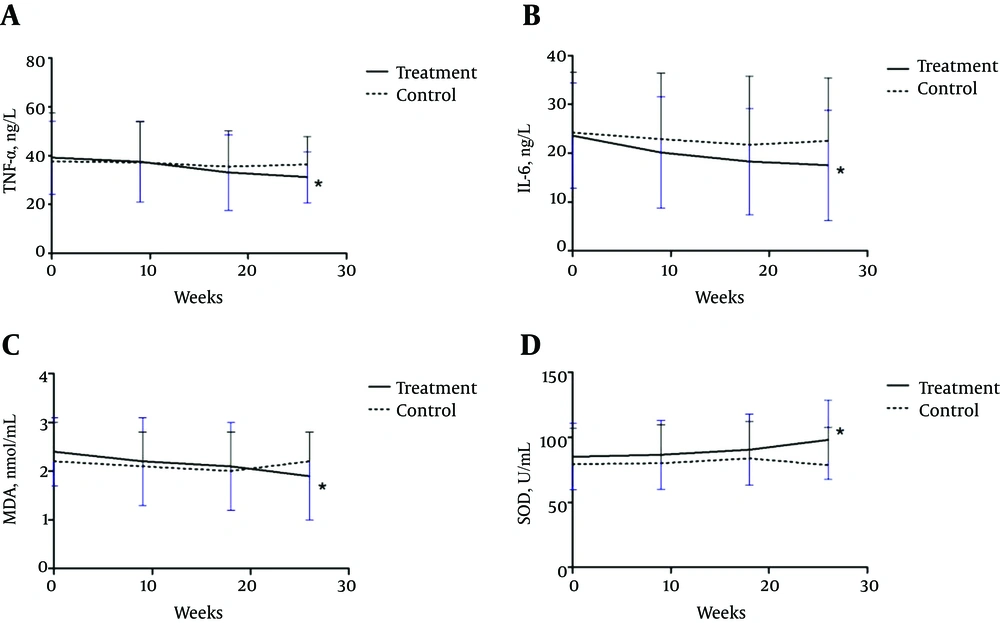
SOD, GPX, MDA, high sensitivity CRP, IL-6, and TNF-α concentration before and after intervention are shown in Table 2. Before starting study, there were no differences in these indices between the JYTK/metformin combination treatment and metformin only groups (P > 0.05). After 18 weeks, serum TNF-α and IL-6 levels decreased by 15.56% and 22.45%, respectively, compared to the baseline values in patients treated with JYTK. By the end of the study, TNF-α, IL-6, and MDA concentrations had decreased by 20.4%, 25.8%, and 20.8%, respectively in the treatment group, as compared to 3.4%, 7.8%, and 0% in the metformin only group (Figure 2). On the other hand, SOD level had increased by 15.27% compared to the control group. There were no differences between the two groups in terms of GXP and CRP levels.
| Groups | Baseline | 9 Weeks | 18 Weeks | 26 Weeks | Variable Change 95% CI |
|---|---|---|---|---|---|
| TNF-α, ng/L | |||||
| treatment group | 39.2 ± 14.9 | 37.6 ± 16.6 | 33.1 ± 15.5b | 31.2 ± 10.5c,d | -8.0 (-12.7, -3.3) |
| control group | 37.7 ± 19.8 | 37.2 ± 16.7 | 35.5 ± 14.6 | 36.4 ± 11.5 | -1.3 (-7.5, 4.9) |
| IL-6, ng/L | |||||
| treatment group | 23.6 ± 10.8 | 20.1 ± 11.4 | 18.3 ± 10.9b | 17.5 ± 11.3c,d | -6.1 (-10.1, -2.1) |
| control group | 24.2 ± 12.4 | 22.9 ± 13.5 | 21.7 ± 14.1 | 22.5 ± 12.9 | -1.7 (-6.6, 3.2) |
| hs-CRP, mg/dL | |||||
| treatment group | 0.13 ± 0.09 | 0.12 ± 0.11 | 0.11 ± 0.10 | 0.10 ± 0.09 | -0.03 (-0.06, 0.00) |
| control group | 0.14 ± 0.08 | 0.13 ± 0.09 | 0.13 ± 0.08 | 0.14 ± 0.07 | 0.0 (-0.03, 0.03) |
| MDA, nM/mL | |||||
| treatment group | 2.4 ± 0.7 | 2.2 ± 0.9 | 2.1 ± 0.9 | 1.9 ± 0.9b,d | -0.5 (-0.8, -0.2) |
| control group | 2.2 ± 0.8 | 2.1 ± 0.7 | 2.0 ± 0.8 | 2.2 ± 0.6 | 0.0 (-0.3, 0.3) |
| SOD, U/mL | |||||
| treatment group | 85.1 ± 25.6 | 86.5 ± 26.7 | 90.6 ± 27.3 | 98.1 ± 30.4b | 13 (2.8, 23.3) |
| control group | 79.3 ± 27.9 | 80.1 ± 29.8 | 83.8 ± 28.4 | 78.5 ± 29.3 | -0.8 (-11.8, 10.2) |
| GPX, U/mL | |||||
| treatment group | 221.6 ± 139.9 | 225.8 ± 114.2 | 250.2 ± 101.3 | 252.7 ± 105.9 | 31.1 (-14.2, 76.4) |
| control group | 198.6 ± 115.7 | 201.5 ± 103.5 | 190.6 ± 107.8 | 205.1 ± 110.4 | 6.5 (-37.1, 50.1) |
Serum Oxidative Stress and Inflammation Parameters in the Subjects With T2DM at Baseline and After JYTK Treatmenta
5. Discussion
JYTK has antioxidant and anti-inflammatory properties; hence, using it in combination with metformin may not only improve glycemia and insulin sensitivity in patients with T2DM, but may also modulate lipid equilibrium. The present study investigated the effect of this combined treatment on serum oxidative stress and inflammation in patients with T2DM. The results indicated that consuming 4.5 g JYTK tablets daily for 26 weeks alters expression levels of oxidative stress and inflammation biomarkers; SOD level increased, while MDA, IL-6, and TNF-α levels decreased as compared to those of the patients treated with metformin alone.
Metformin along with pioglitazone, statins, glimepiride, voglibose, amlodipine, and telmisartan are widely used to treat diabetes. In the past several years, traditional Chinese medicine, which involves combining different herbs into a single therapy, has improved diabetic symptoms and complications (18-21), possibly through antioxidant and anti-inflammatory mechanisms (22). The increase in free radical generation in patients with T2DM can lead to vascular changes (23, 24); Panax ginseng, grape seed extract, and bitter melon all exhibit antioxidant capacities (23, 25-26), while diosgenin present in fenugreek improves glucose metabolism by promoting adipocyte differentiation and inhibiting inflammation in adipose tissues (27). TangBiKang, a traditional Chinese medicine granule, prevents diabetic peripheral neuropathy-induced increases in serum IL-6 and TNF-α levels (28).
JYTK tablets contain ZhiMu, GuiJianYu, and CiWuJia as well as polysaccharides, flavonoids, mangiferin, anemaran, steroidal saponins, bis (pyridine-2-yl) ketone, and other active ingredients. Polysaccharides and flavonoids in Guijianyu demonstrate oxygen free radical-scavenging activity towards phenolic compounds (3-5), while R. anemarrhenae has anti-diabetic effects, with mangiferin inhibiting the activity of α-glucosidase. Anemaran suppresses acute and chronic inflammation by inducing the secretion of adrenal glucocorticoid and inhibiting prostaglandin E synthesis or releasing in the inflammatory tissues (6, 7). Thorn thistle saponins can reduce blood glucose levels in rats possibly by increasing antioxidative enzyme activity, which reduces cellular damages caused by free radicals and suppresses the immune response (8, 9). Fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1C) level, and a lipid profile were measured before, during, and after the combined JYTK/metformin treatment. It was found out that the combination treatment not only helped to improve glycemia and insulin sensitivity, but it also helped to modify the diabetes related lipid equilibrium (14). The results of this study suggest that combined JYTK/metformin treatment may be effective to prevent diabetes complications resulted from increased inflammation, vasculogenesis, and oxidative stress.
This study had some limitations. Firstly, the levels of CAT or other inflammatory factors such as IL-1 were not measured. Secondly, the sample size was relatively small and the intervention period was only 26 weeks, and it is therefore unclear whether JYTK/metformin treatment can lead to long-term improvement in patients with T2DM.
In summary, this study provided evidence that JYTK/metformin treatment can improve antioxidant indices and reduce inflammation in patients who are newly diagnosed with T2DM. Further investigations with larger sample sizes and longer intervention time are needed to confirm the beneficial combination effects of JYTK in the management of diabetes complications. Elucidation of the mechanistic basis for these effects requires further study.