1. Background
Postmenopausal osteoporosis is a skeletal disorder in which the bone mass is decreased and micro architecture of the bone is weakened. It is a major cause of morbidity in postmenopausal women and obviously estrogen deficiency plays a key role in bone loss and fragility in this group. However, recently it has been shown that several cytokines and immune factors are involved in the pathogenesis of postmenopausal osteoporosis, and thus it can be considered as an immune disease (1, 2).
Throughout life, bone remodeling continues as a result of osteoblasts and osteoclasts functions and interactions. Osteoblasts are generated from stem cells in the bone marrow, called Mesenchymal stem cells (MSCs), whereas osteoclasts arise from hematopoietic stem cells (HSCs). An exquisite balance is needed in osteoblast and osteoclast functions in order to maintain bone architecture and strength (3, 4).
The transforming growth factor (TGF)-β molecules are a super family of cytokines, including TGF-β, bone morphogenetic proteins (BMPs) and activins, which regulate the growth and differentiation of osteoblasts and osteoclasts (5). The TGF-β1, TGF-β2 and TGF-β3 are closely related mammalian isoforms, with TGF-β1 being the most abundant isoform in the bone (6). There is accumulating evidence demonstrating the role of TGF-β1 in bone development, mineral storage and generation of hematopoietic cells. The TGF-β1 augments proliferation and inhibits apoptosis of osteoblasts, and is a chemoattractant molecule for osteoblast precursors (MSCs) (7, 8). However, there are studies demonstrating that TGF-β1 inhibits osteoblast proliferation and mineralization in later phases, and in late phase of their differentiation, osteoblasts seem to be less sensitive to TGF-β1, and in final differentiation stages (mature osteoblasts) TGF-β1 opposes BMP actions (9, 10). Transforming Growth Factor-β1 has a complex impact on osteoclasts, and recruits them from bone marrow and spleen to the bone, enhances their maturation but also induces their apoptosis (11, 12). There are studies demonstrating paradoxical effects of TGF-β1 on osteocalst differentiation, in concordance to its concentration. Lower levels of TGF-β1 promote differentiation while higher doses restrain differentiation of osteoclasts (13, 14).
More than a few studies tried to clarify the role of TGF-β1 in the pathogenesis of osteoporosis. In a study conducted by Akinci et al. (15) on males with osteoporosis, they demonstrated that TGF-β1 level is decreased in the sera of these cases. In a rat model, Estai et al. (5) suggested that treatment with estrogen improved the strength of the bone in estrogen-deficient rats by inducing the expression of TGF-β1. In another study by Kim et al. (16), it was implied that TGF-β1 positively inhibits bone resorption in rats. In contrary to these studies, Georgescu et al. (17) stated that there is no evidence for a major role of TGF-β1 as a determinant of bone mass in osteoporotic males or postmenopausal females.
Interleukin 18 (IL-18) is another molecule, the impacts of which on the osteoclasts need more investigation. There are studies reporting that IL-18 inhibits osteoclastic formation and bone resorptive activity (18, 19), while others demonstrate that IL-18 can stimulate osteoclast formation indirectly (20, 21).
2. Objectives
Transforming growth factor-β1 and IL-18 play complex roles in normal bone metabolism, and in pathophysiology of postmenopausal osteoporosis. The major goal of this study was to compare the TGF-β1 and IL-18 levels in the sera of postmenopausal females with and without osteoporosis. Besides, we tried to demonstrate if there is any association between the level of these molecules in the serum and the clinicopathological features of postmenopausal osteoporotic females, such as age, body mass index (BMI), parity, smoking and vitamin D supplementation.
3. Methods
3.1. Study Design, Participants and Data Collection
An analytic cross sectional study was conducted in order to further clarify the role of TGF-β1 and IL-18 in osteoporosis of postmenopausal females. The study included 65 postmenopausal females with osteoporosis as cases and 69 postmenopausal females of similar age without osteoporosis as controls. The cases and controls were matched for age and BMI. Participants were recruited from the gynecology department of a hospital affiliated with Shiraz University of Medical Science, and were included in the study if they had had amenorrhea for at least one year.
Dual energy X-ray absorptiometry (DXA) (Lunar Corporation, Madison, Wisconsin, USA) of lumbar spine and femoral neck was used to determine bone mass density (BMD) of participants and T-scoring was applied to establish whether the patient has osteoporosis or not. T-scoring determines the number of units (standard deviation) the bone density is below or above the average bone density of a 30-year-old healthy adult with the same gender (22). Patients were considered osteoporotic if their bone density was 2.5 SD or more below the young adult mean (T-score < -2.5) and participants with a T-score of -1 and above were considered to be normal in bone mass densitometry and were selected as controls. Osteopenic individuals (-2.5< T-score <-1) were not included in this study.
Demographics of the participants (including age, weight and height) and other variables (including age of menarche, age of menopause, parity, smoking, calcium and vitamin D consumption) were collected through a complete interview and physical examination and they are summarized in Table 1. Patients with a history of hysterectomy, unilateral/bilateral oophorectomy, atherosclerotic vascular diseases, diabetes mellitus, familial hyperlipidemia, severe and chronic systemic diseases, chronic hypertension, endocrine diseases and immune deficiency were excluded from the study. The patients with metabolic bone diseases or any secondary cause of osteoporosis such as thyroid and parathyroid diseases, malignancies, hypercortisolism (primary or secondary) and those on steroids, heparin and chemotherapy, were also excluded.
Abbreviations: BMI, body mass index; TGF-β1, transforming growth factor β1; IL-18, interleukin 18.
aMean ± standard deviation (SD).
bIndependent sample t-test.
cMedian.
dMann-Whitney U test.
eStatistically significant (P < 0.05).
All individuals included in this study were informed and their consent to participate was taken in written form. Declaration of Helsinki was considered in this study and the research protocol was approved by the ethics committee of Shiraz University of Medical Science.
3.2. Preparation of Blood Sample and Assay for Transforming Growth Factor-β1 and Interleukin-18
Blood samples were drawn from 134 participants and were collected in sterile clean, dry tubes. Sera were rapidly separated after coagulation and were kept frozen at -70°C until time of assay for IL-18 and TGF-β1. Serum TGF-β1 and IL-18 level was measured by the commercial enzyme linked immunosorbent assay (ELISA) kit (R&D systems, Abingdon, UK), according to the manufacturers’ guidelines. The sensitivity of the assay for TGF-β1 was 15.4 pg/mL and for IL-18 was 25 pg/mL. In order to evaluate intra-assay precision, five serum samples of known concentration (R&D Systems’ Quantikine® kits, Abingdon, UK) were tested on one plate. The average of intra-assay coefficient of variability (CV) for TGF-β1 was 5.2% and for IL-18 was 5.7%. Five samples, of known concentration, were tested in separate assays to assess inter-assay precision, and average of high and low control for TGF-β1 and IL-18 was 6.1% and 5.8%, respectively.
3.3. Statistical Methods
We used statistical package for social science (SPSS) 17 for data analysis. In order to determine if the variables are normally distributed or not, we used the Shapiro-Wilk test. Data with normal distribution were presented as mean ± standard deviation (SD), and otherwise as median. Mann-Whitney U test and Kruskal-Wallis test was used to compare the difference between groups. Pearson correlation was utilized to correlate bivariates. Two-tailed P < 0.05 was considered statistically significant.
4. Results
A total of 134 post-menopausal females, who were referred to the department of gynecology of Shiraz University hospitals, participated in this study. Sixty-five of the participants were osteoporotic in bone mineral densitometry. Mean age of the participants was 53.5 ± 6.8 years and mean of menopause age was 47.5 ± 4.1 years. The mean BMI in both cases and control groups was 26.5 ± 4.0 kg/m2.
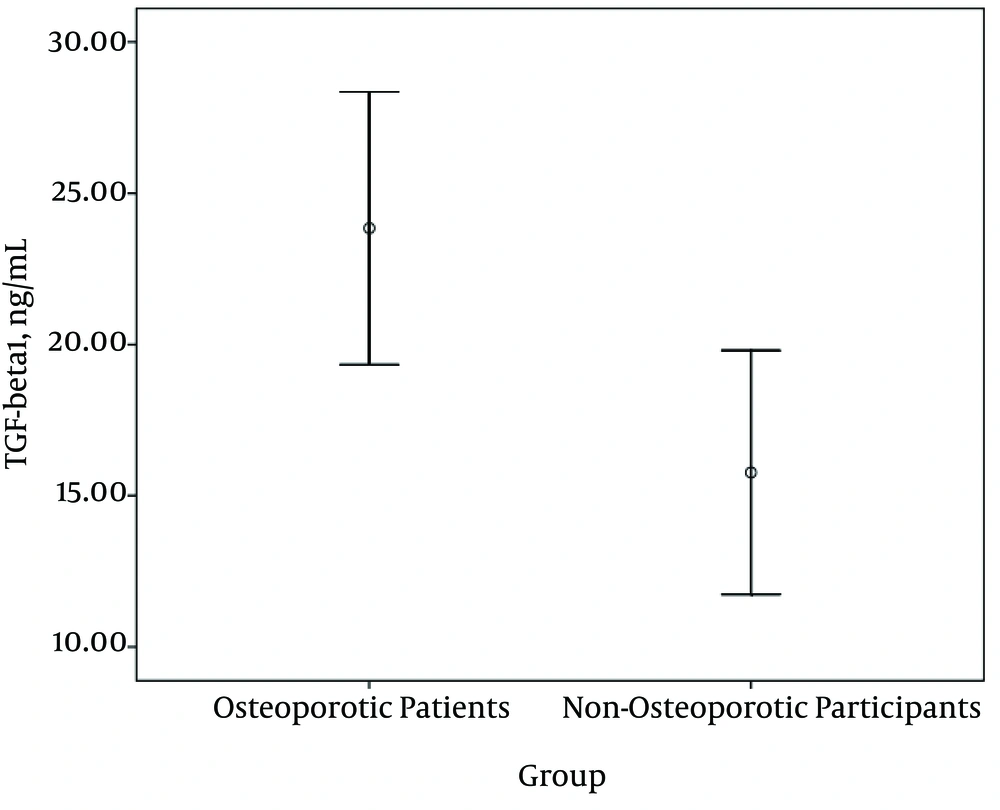
As the TGF-β1 and IL-18 levels in the sera of both cases and controls were not normally distributed (Shapiro-Wilk test, P = 0.00), Mann-Whitney U test was employed to compare the circulating levels of TGF-β1 and IL-18 between these two groups. Serum TGF-β1 levels were significantly higher in osteoporotic postmenopausal females than non-osteoporotic individuals (Figure 1; 23.8 vs. 15.8 ng/mL; P = 0.009).
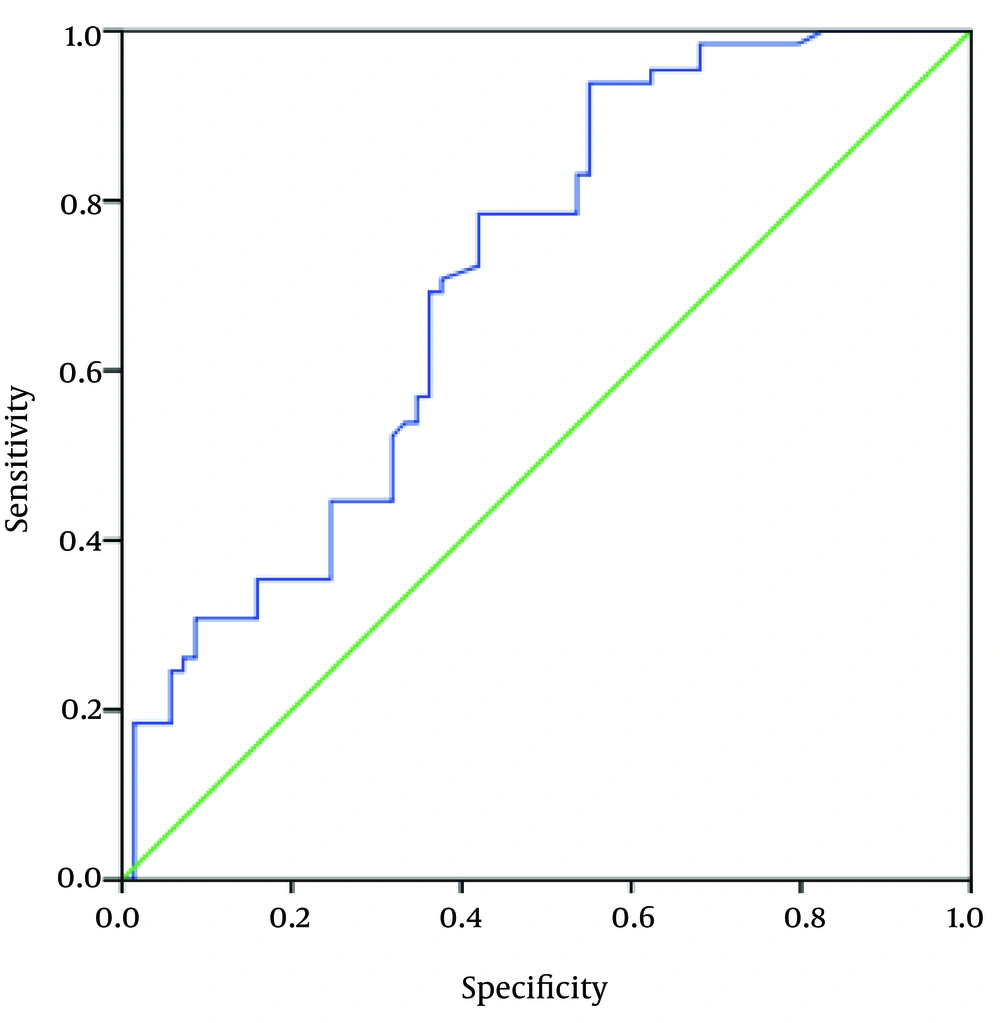
Receiver operating characteristic (ROC) curve analysis was established to further investigation of the accuracy of TGF-β1 levels to discriminate patients with osteoporosis from control subjects. Area under the curve for TGF-β1 was 0.71 (Figure 2; P = 0.04). This means on average, an osteoporotic postmenopausal female will have a higher circulating TGF-β1 level than 71% of the non-osteoporotic postmenopausal females.
There was a positive correlation between TGF-β1 serum levels and BMI in osteoporotic postmenopausal females and the serum levels of TGF-β1 were significantly higher in participants with BMI > 25 kg/m2 (Table 2), 16.4 vs. 25.4 ng/mL; P = 0.04). No other statistically significant correlation was found between circulating TGF-β1 levels and age, age of menarche or menopause, parity, smoking, vitamin D and calcium consumption in both case and control groups.
| Variable | No. (%) | Tgf-β1, (ng/mL)a | P Value |
|---|---|---|---|
| Smoker | 0.76b | ||
| Yes | 22 (33.85) | 26.09 | |
| No | 43 (66.15) | 22.70 | |
| Calcium-vitamin D supplementation | 0.69b | ||
| Yes | 20 (30.80) | 24.83 | |
| No | 45 (69.20) | 23.38 | |
| Body mass index (BMI), kg/m2 | 0.04b,c | ||
| BMI < 25 | 11 (16.92) | 16.42 | |
| BMI > 25 | 54 (83.08) | 25.35 | |
| Parity | 0.43d | ||
| 0 | 7 (10.77) | 21.97 | |
| 1 | 3 (4.62) | 20.04 | |
| 2 | 10 (15.38) | 25.76 | |
| > 3 | 45 (69.23) | 23.96 |
aMedian.
bMann-Whitney U test.
cStatistically significant (P < 0.05).
dKruskal-Wallis test.
No statistically significant difference between IL-18 levels in the sera of cases and controls was found. Also we found no significant correlation between serum levels of IL-18 and TGF-β1, age of the participant, age of menopause or menarche, parity, tobacco use, parity and consumption of calcium and vitamin D supplements, in both groups. Neither IL-18 levels nor TGF-β1 levels were related to the severity of osteoporosis in the patient group.
5. Discussion
In this study, we demonstrated that TGF-β1 levels in the sera of postmenopausal osteoporotic females were significantly higher than non-osteoporotic postmenopausal controls. Our observation opposes the results of several studies that have reported TGF-β1 has a protective role against osteoporosis and aids bone healing (3-5, 7, 11, 22). However, there are several other studies consistent with our results. Cheng et al. (23) suggested that TGF-β1 levels increase during postmenopausal period and decrease during old age. Most of the osteoporotic patients in our study were in their early menopausal phases. Also, in a rodent study conducted by Ota et al. (24), it was demonstrated that TGF-β1 increases osteoclast production and cultures with suppressed TGF-β activation keep their capacity to raise mineralization.
The impact of immune system on bone formation and remodeling has led to considering osteoporosis as a chronic immune disease (25). In postmenopausal females, aging and estrogen deficiency are the major cause of decreased bone mineral density. Many estrogen-dependent growth factors and cytokines are involved in bone remodeling such as IL-1, IL-6, insulin-like growth factor I and II, colony stimulating factor, osteoprotegrin and TGF-β (26, 27). Transforming Growth Factor-β1 has been shown to interact with hormones and soluble factors, like estrogen and vitamin D and there are studies demonstrating that estrogen stimulates TGF-β1 production, and promotes osteoblast proliferation and maturation. Also, estrogen causes osteoclast apoptosis through a TGF-β-dependent pathway (28).
Balooch et al. (29) demonstrated that increased TGF-β signaling in genetically modified mouse models, causes decreased mineral component of the bone and weakened mechanical properties. In a rodent model of osteoarthritis, Zhen et al. (30) demonstrated that activated TGF-β1 is increased in subchondral bone, which later becomes osteoporotic. In human beings, high levels of active TGF-β1 cause several skeletal disorders as a result of abnormalities in bone remodeling. Camurati-Engelmann disease (CED) is caused by mutation in TGF-β1 gene, resulting in premature activation of this protein. In the histology bone samples of these patients, trabecular connectivity is decreased despite normal osteoblast and osteoclast numbers. This may be suggestive of uncoupled bone remodeling caused by hyperactive TGF-β1 in CED patients (31, 32).
Aging leads to dramatic bone loss, especially in females during the postmenopausal years and osteoporosis is the result of osteoclast bone resorption being more than osteoblast bone formation. Previously, we mentioned that osteoblasts originate from MSCs. With aging, MSCs also experience senescence and their proliferative capacity and consequently bone formation is decreased (33). As these cells age, they start secreting various factors, including TGF-β1 (34). Also during aging, the concentration of reactive oxygen species (ROS) increases in the bone microenvironment, decreasing osteoblast differentiation and increasing osteoclast activity and releasing more TGF-β1 (35, 36). Transforming Growth Factor-β1 is the second important chemoattractant molecule for osteoclasts and monocytes after M-CSF (12), and bone resorption is accompanied by higher levels of circulating TGF-β1 (37).
Transforming growth factor-β1 released by osteoclasts induces the migration of MSCs to bone resorption sites for osteoblast differentiation and bone formation. However, as the MSCs are old and their capacity to proliferate and differentiate is decreased, elevated TGF-β1 levels leads to recruitment and activation of more osteoclasts and bone resoption outmatches bone formation, which leads to osteoporosis (4).
There was no difference between IL-18 levels in the sera of osteoporotic and non-osteoporotic postmenopausal females in this study. Although in a study conducted by Morita et al. (20), it was demonstrated that IL-18 could inhibit tumor necrotizing factor (TNF)-α induced osteoclastogenesis in mice. In contrary, Dai et al. (21) stated that IL-18 can indirectly stimulate osteoclast formation by up-regulating RANK Ligand production in T-cells of rheumatoid arthritis patients’ synovium. In another study, Maugeri et al. (38) showed that 12 months after treating post-menopausal osteoporotic females with bisphosphonates, BMD and circulating IL-18 were increased. Even though our survey involved relatively small groups, it did not allow us to state that IL-18 should not be considered as a marker of bone resorption, and further investigations are needed. In our study, age, age of menopause and menarche, parity, consumption of calcium and vitamin D had no impact on serum levels of TGF- β1 and IL-18.
This study design did not allow us to derive an etiologic conclusion that the observed higher level of TGF-β1 in osteoporotic females is the cause of bone resorption. We selected our participants from a population of patients referred to the gynecology department, since cases and controls were not derived from a prospective cohort study and nor from the general population thus we should consider this limitation in future studies to better understand the role of TGF-β in pathogenesis of osteoporosis. Our participants were in early postmenopausal ages thus a follow up study with a larger sample size will allow us to better elucidate the role of TGF-β in a time trend analysis.
In conclusion, our study demonstrated that TGF-β1 serum levels is higher in osteoporotic postmenopausal females than non-osteoporotic ones, and probably aberrant increase in TGF-β1 in postmenopausal females can result in uncoupled bone resorption and formation, which leads to osteoporosis. Additional investigations are needed to further clarify the role of TGF-β1 in postmenopausal osteoporosis.