1. Background
Obesity is a major public health problem worldwide. Obesity has emerged as an epidemic issue accompanied by a variety of health complications (1), including cardiovascular diseases (2), diabetes mellitus (3), asthma (4), arthritis, Alzheimer’s disease (5), and cataract (6). Based on a report by the World Health Organization, obesity is a worldwide health concern given that the prevalence of over-weight and obesity is now 60% of the population (7). In the STEPS Survey in 2011, the prevalence of obesity in Iranian adults was reported to be as high as 22.3% (1).
Several WL strategies such as medical, dietary management, and surgical procedures have been developed and experimented over the past decades in order to improve health (7, 8). As previously mentioned, decreasing calorie intake and increasing physical activity are recommended for WL (9). These strategies are based on distribution of macronutrient such as low-carbohydrate/high-fat, high-carbohydrate/low-fat (10), or low-carbohydrate/high protein diet (11), or rate of WL including slow or rapid WL (7).
It is clearly marked that obesity is defined as an excess accumulation of body fat but not only excess body weight (12, 13). Therefore, fat mass (FM) measurement is the best way to determine obesity and its classification. However, in the past decades, the three most common ways of assessing obesity were weight, body mass index (BMI), and waist circumference (WC) instead of measuring body FM. In addition, it has been recognized that abdominal obesity, assessed by WC, predicts obesity-related health risks and an accumulating body of evidence indicates that compared to BMI alone, WC along with BMI better predicts health risk. Janssen et al. (14), suggest that WC is a better indicator of health risk compared to BMI, and consequently it should be much more emphasized on WC in the obesity classification system. To date, other indices have been suggested as a potential determinant of obesity including a body shape index (ABSI) (15), body adiposity index (BAI) (16), waist to hip ratio (WHR) (17), BMI z score, BMI percentage, and waist to height ratio (WHtR) etc.
WHtR is a simple and rapid screening tool, which can help overcome debates about the use of different BMI categories for evaluating health risks in different populations (18). WHtR was first used in the Framingham study (19). It has been used for determining abdominal obesity and is related to the progression of diabetes mellitus as well as risk factors for cardiovascular diseases (20, 21).
2. Objectives
To our knowledge, the current study is the first study comparing obesity-assessing indices such as BMI, WC, WHtR, ABSI, BAI etc. with body FM before and after intentional WL in different rates to show, which indices can be optimal to estimate the rate of FM changes in WL programs. On the other hand, the aim of this study is to determine which index has the strongest relationship with fat mass during WL regardless of its rates. In addition, this clinical trial study was to evaluate the effects of anthropometric indices of the two protocols on WL in obese and overweight people.
3. Methods
3.1. Subjects
A total of 68 subjects with overweight and/or obesity (25% males) who had a BMI > 25 and/or percentage of body fat of more than 25% for men and 35% for women (22) were enrolled in a double-blind clinical trial study. All subjects had no history of cardiovascular diseases, inflammatory (e.g. rheumatoid arthritis, hepatitis, asthma) and auto-immune disease, diabetes mellitus, thyroid disease, hormonal replacement therapy, corticosteroids therapy, or medication that could affect weight and/or body composition. Disease history information at baseline was collected during a face-to-face personal interview conducted in a study center by trained interviewers. In addition, they had a sedentary lifestyle and were non-pregnant, non-lactating, non-smoker, non-alcohol consumers, and without any history of dietary or exercise intervention in the last year. All subjects gave written informed consent, and the protocol was approved by the ethical committee of Ahvaz Jundishapur University of Medical Science (Ethic code: IR.AJUMS.REC.1394.212).
3.2. Weight Loss Protocol
The weight loss protocol was performed as previously described (23). Prior to WL, an ambulatory run-in period was imposed for each subject to ensure stabilization of body weight (± 2 kg during 4 weeks). During the body weight stabilization, a 3-day dietary food record was used to determine each subject’s daily food and beverage intake to estimate their total daily caloric intake. Thereafter, subjects underwent a WL program aiming to reduce the initial body weight by at least 5% and a maximum 10%. Rapid and slow WL have been defined based on the weight lost over a period of 5 and 15 weeks, respectively in order to decrease weight, the prescribed low calorie diet contained 15% of the total calorie as protein, 30% - 35% as fat, and 50% - 55% as carbohydrate. In general, the meal plans included three main meals (breakfast, lunch, and dinner) and three snacks (mid-morning, mid-afternoon, and bedtime); in addition, they were low in saturated and trans fats, cholesterol, salt (sodium), and added sugars. All diets were designed according to the dietary guidelines for Americans 2010 (9). Low-calorie diets provided an energy deficit of 500 - 750 and 1000 - 1500 Kcal per day for slow and rapid weight loss, respectively. At the end of the study, anthropometric assessments have been conducted on individuals and they were divided into two groups randomly (34 individuals in rapid WL and 34 individuals in slow WL). The follow up of the subject to adhere the dietary prescription was weekly and performed using telephone and face-to-face interviews.
3.3. Energy Intake
Subjects were provided with a food scale and instructed on how to complete a 3-day dietary record. Caloric intake was calculated using a 3-day food diary (two weekdays and one weekend day). Caloric intake from each food record was calculated, using the Nutritionist IV nutrient analysis software (first data bank, The Hearst Corporation, San Bruno, Calif).
3.4. Body Composition
Total and regional FM, lean body mass (LBM), and total body water were measured using direct segmental multi-frequency bioelectrical impedance method (inbody 270, Biospace, Korea) (24). Measurements were undertaken in a fasted state, readily after waking in a euhydrated state. WC was measured in a standing position at the level of the noticeable waist narrowing located approximately half way between the costal border and the iliac crest. All Anthropometric parameters were conducted in triplicates and the mean value was calculated for each subject.
3.5. Resting Metabolic Rate
RMR was measured at baseline and following dietary Intervention by indirect calorimetry (FitMate, Cosmed, Rome, Italy) using the resting oxygen uptake (VO2).
3.6. Indices Calculation
The BMI is defined as the body mass divided by the square of the body height and was determined as kg/m2. ABSI was determined based on a formula that developed previously: ABSI = WC/(BMI2/3 height1/2). To control age and sex differences in mean ABSI, we entered it into proportional hazards regression for mortality as a z score (15):
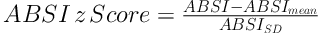
BMI z score and BMI percentage were calculated according to WHO technical report (25). BAI was calculated by the size of the hips compared to the person’s height (16) (BAI = ((hip circumference)/((height)1.5)-18)). Body-surface area (BSA) also calculated based on Mosteller method (26). The FFM and FM indices are equivalent concepts to the BMI, as shown in Equations 2 and 3 (27):
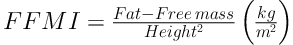
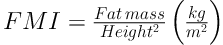
Note that, mathematically, BMI (kg/m2) = FFMI (kg/m2) + FMI (kg/m2).
3.7. Statistical Analysis
Statistical analyses were conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). The data were checked for normality using Kolmogorov-Smirnov test. Independent sample t-test (for normally distributed variables) and Mann-Whitney U test (for non-normally distributed variables) were used to compare baseline values between the two groups. Paired sample t-test was used to compare significance before and after intervention within groups. Between-group comparison of changes was tested by Independent-samples Student’s t-test analysis. To investigate the association between mean value of parameters such as percentage body fat, ABSI, BSA, BMI, hip circumference, WC, and weight, partial correlation analyses were performed, controlling for age and gender. Data are reported as the mean ± standard error. The P < 0.05 was considered significant.
4. Results
There were no significant differences in the baseline characteristics of the groups in total energy, age, weight, BMI, other anthropometric measures, resting systolic and diastolic blood pressure, and dietary intake between groups (Table 1). As shown in Table 2, there were no significant differences in weight loss and BMI (P > 0.05). However, the reduction in total daily calorie intake was significantly higher in rapid WL compared to slow WL group (P < 0.001). The WC and HC were significantly reduced more in slow WL versus rapid WL (P < 0.001). Along with the more reduction in FFM in rapid WL group, the LBM reduction was significantly lower in slow WL group compared with the rapid WL group (P < 0.001).
| Slow WL, N = 34 | Rapid WL, N = 34 | P Value | |
|---|---|---|---|
| n (male) | 34 (9) | 34 (8) | 0.783 |
| Age (years) | 35.1 ± 9.3 | 34.6 ± 10.3 | 0.845 |
| Weight (kg) | 87.0 ±14.4 | 85.4 ± 15.4 | 0.652 |
| Height (cm) | 162 ± 7.4 | 162 ± 9.4 | 0.770 |
| BMI (kg/cm2) | 33.6 ± 6.3 | 32.5 ±6.4 | 0.481 |
| Total daily calorie intake (Kcal/day) | 2441 ± 405 | 2619 ± 472 | 0.100 |
| WC (cm) | 99.1 ± 13.0 | 98.3 ± 13.4 | 0.787 |
| HC (cm) | 103.2 ± 8.8 | 103.4 ± 8.5 | 0.921 |
| WHR | 0.95 ± 0.07 | 0.94 ± 0.08 | 0.653 |
| Resting systolic BP (mmHg) | 133.3 ± 13.2 | 131.2 ± 15.3 | 0.554 |
| Resting diastolic BP (mmHg) | 85.4 ± 8.2 | 82.0 ± 8.2 | 0.094 |
| Heart rate (beat/min) | 88.9 ± 11.6 | 93.0 ± 13.1 | 0.181 |
| LBM(kg) | 28.1 ± 6.1 | 28.1 ± 6.6 | 0.994 |
| LBM% | 32.3 ± 4.4 | 33.0 ± 5.6 | 0.537 |
| FM (kg) | 36.6 ± 9.9 | 34.9 ± 12.0 | 0.528 |
| PBF (%) | 41.8 ± 7.3 | 40.4 ± 9.2 | 0.493 |
| FM/LBM | 1.3 ± 0.3 | 1.3 ± 0.4 | 0.659 |
| TBW (kg) | 36.9 ± 7.2 | 37.1 ± 7.9 | 0.937 |
| FFM (kg) | 50.4 ± 9.9 | 50.4 ± 10.7 | 0.984 |
| RMR (kcal) | 1605 ± 235 | 1607 ± 256 | 0.971 |
| Arms LBM (kg) | 5.6 ± 1.5 | 5.6 ± 1.7 | 0.988 |
| Trunk LBM (kg) | 23.3 ± 4.5 | 23.4 ± 5.0 | 0.910 |
| Feet LBM (kg) | 15.2 ± 3.1 | 15.0 ± 3.2 | 0.828 |
| Arms FM (kg) | 6.4 ± 2.8 | 6.1 ± 3.7 | 0.738 |
| Arms FM (%) | 50.5 ± 11.6 | 48.2 ± 14.9 | 0.489 |
| Trunk FM (kg) | 18.2 ± 3.9 | 17.4 ± 4.6 | 0.456 |
| Trunk FM (%) | 42.3 ± 5.8 | 41.1 ± 7.6 | 0.455 |
| Feet FM (kg) | 10.5 ± 3.3 | 9.9 ± 3.7 | 0.489 |
| Feet FM (%) | 39.2 ± 8.1 | 37.9 ± 10.0 | 0.558 |
Abbreviations: BMI: body mass index, BP, blood pressure; FFM, fat free mass; FM, fat mass; HC, hip circumstance; LBM, lean body mass; PBF: percent body fat; RMR, resting metabolic rate; TBW, total body water; WC, waist circumstance; WHR: waist to hip ratio; WL: weight loss.
| Δ Post-Baseline | |||
|---|---|---|---|
| Slow WL (Within Group P*), n = 34 | Rapid WL (Within Group P*), n = 34 | P Value** | |
| Weight loss (%) | 6.1 ± 1.0* | 5.9 ± 0.9* | 0.436 |
| Weight loss (kg) | -5.3 ± 1.2* | -5.0 ± 1.2* | 0.324 |
| BMI (kg/cm2) | -2.0 ± 0.4* | -1.9 ± 0.5* | 0.321 |
| Reduction in total daily calorie (kcal) | 638 ± 64* | 1207 ± 133* | < 0.001 |
| WC (cm) | -6.3 ± 1.4* | -4.6 ± 1.5* | < 0.001 |
| HC (cm) | -4.8 ± 1.7* | -3.2 ± 1.7* | < 0.001 |
| WHR | -0.1 ± 0.01* | -0.1 ± 0.01* | 0.930 |
| Resting systolic BP (mmHg) | -3.1 ± 10.3 | -3.3 ± 11.1 | 0.943 |
| Resting diastolic BP (mmHg) | -1.2 ± 7.0 | 0.5 ± 7.3 | 0.406 |
| Heart rate (beat/min) | 3.2 ± 10.5 | -0.6 ± 12.1 | 0.253 |
| LBM (kg) | -0.3 ± 0.6* | -1.4 ± 0.6* | < 0.001 |
| LBM% | 1.6 ± 0.9* | 0.2 ± 0.8 | < 0.001 |
| FM (kg) | -4.7 ± 1.4* | -2.8 ± 1.4* | < 0.001 |
| PBF (%) | -3.1 ± 1.5* | -1.0 ± 1.3* | < 0.001 |
| FM/LBM | -0.15 ± 0.06* | -0.04 ± 0.06* | < 0.001 |
| TBW (kg) | -0.4 ± 0.8* | -1.7 ± 0.7* | < 0.001 |
| FFM (kg) | -0.6 ± 1.0* | -2.1 ± 1.0* | < 0.001 |
| RMR (kcal) | -160 ± 34* | -197 ± 38* | < 0.001 |
| Arms LBM (kg) | -0.1 ± 0.2* | -0.3 ± 0.4* | 0.022 |
| Trunk LBM (kg) | -0.3 ± 0.7* | -0.9 ± 0.5* | < 0.001 |
| Feet LBM (kg) | -0.3 ± 0.4* | -0.4 ± 0.2* | 0.147 |
| Arms FM (kg) | -1.2 ± 0.4* | 0.7 ± 0.6* | 0.001 |
| Arms FM (%) | -4.8 ± 2.5* | -1.6 ±1.9* | < 0.001 |
| Trunk FM (kg) | -2.0 ± 1.1* | -1.3 ± 0.7* | 0.006 |
| Trunk FM (%) | -2.6 ± 1.6* | -1.1 ± 1.2* | < 0.001 |
| Feet FM (kg) | -1.4 ± 0.8* | -0.6 ± 0.5* | < 0.001 |
| Feet FM (%) | -2.9 ± 1.4* | -0.9 ± 1.1* | < 0.001 |
Abbreviations: BMI, body mass index; BP, blood pressure; FFM, fat free mass; FM, fat mass; HC, hip circumstance; LBM, lean body mass; PBF, percent body fat; RMR, resting metabolic rate; TBW, total body water; WC, waist circumstance; WHR, waist to hip ratio; WL, weight loss.
a* Significant changes within groups: P ≤ 0.05, ** between groups P.
As shown in Table 3, there were no significant differences in BSA, ABSI, BMI z score, and BMI percentage between groups (P > 0.05). However, the mean reduction in BAI percentage, WHtR, FMI, FFMI, ABSI z score, and ABSI percentage were significantly higher in slow WL compared to rapid WL group (P < 0.05). Within group changes, all measures were significant (P < 0.05) except for ABSI in rapid WL group (P > 0.05).
| Δ Post-Baseline | |||
|---|---|---|---|
| Slow WL (Within Group P*), n = 34 | Rapid WL (Within Group P*), n = 34 | P Value** | |
| BAI percentage | -2.3 ±0.8* | -1.5 ± 0.8* | 0.001 |
| BSA | -0.6 ± 0.1* | -0.5 ± 0.1* | 0.308 |
| WHtR | -0.03 ± 0.008* | -0.02 ± 0.009* | < 0.001 |
| FMI (kg/m2) | -4.7 ± 1.4* | -2.8 ± 1.4* | < 0.001 |
| FFMI (kg/m2) | -0.6 ± 1.03* | -2.1 ± 1.01* | < 0.001 |
| ABSI | 0.07 ± 0.2* | 0.02 ± 0.1 | 0.186 |
| BMI z score | -0.3 ± 0.1* | -0.3 ± 0.01* | 0.251 |
| ABSI z score | -0.4 ± 0.3* | -0.1 ± 0.3* | < 0.001 |
| BMI percentage | -7.9 ± 4.1* | -7.8 ± 3.4* | 0.950 |
| ABSI percentage | -10.5 ± 7.5* | -3.4 ± 7.8* | <0.001 |
Abbreviations: ABSI, a body shape index; BAI, body adiposity index; BMI, body mass index; BSA, body-surface area; FFMI, fat free mass index; FMI, fat mass index; WHtR, waist to height ratio; WL, weight loss.
a* Significant changes within groups: P ≤ 0.05, ** Between groups P.
Interestingly, as shown in Table 4, correlations ranged from a high value (r = 1) for FM and FMI, r = 0.986 for WHtR and WC, BAI% and HC to a low value of 0.239 for BSA and WHR. ABSI% was significantly correlated with WC and WHtR; however, there was no significant correlation between ABSI and any of the measurements, except for LBM% (low correlation, r = 0.274, P < 0.05). Nevertheless, after adjusting for age and sex, the significant correlation was found between ABSI-z score and PBF, WC, HC, FM/LBM, FM, FFM, LBM%, WHtR, BAI%. Robust correlation was observed between FM/LBM and PBF (r = 0.918), LBM% and PBF (r = -0.949), LBM% and FM/LBM (r = -0.904), WHtR and WC (r = 0.986), BAI% and HC (r = 0.986) (all P < 0.001). As shown in Table 5, changes in FM and LBM had correlation with changes in arms, trunk, and feet in both groups (P < 0.05, or P < 0.001) except for FM and feet changes in slow group (P > 0.05).
| PBF | BMI | Weight | WC | HC | WHR | FM/LBM | FM | FFM | LBM% | WHtR | BSA | BAI% | ABSI | ABSI% | ABSI- z Score | BMI% | BMI- z Score | FMI | FFMI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBF | 1 | |||||||||||||||||||
| BMI | 0.234 | 1 | ||||||||||||||||||
| Weight | 0.286* | 0.885**,b | 1 | |||||||||||||||||
| WC | 0.645**,c | 0.430** | 0.461** | 1 | ||||||||||||||||
| HC | 0.426** | 0.249* | 0.233 | 0.709**,b | 1 | |||||||||||||||
| WHR | 0.221 | 0.215 | 0.283 | 0.397* | -0.210 | 1 | ||||||||||||||
| FM/LBM | 0.918** | 0.345* | 0.310* | 0.618**,c | 0.391* | 0.223 | 1 | |||||||||||||
| FM | -0.845**,b | -0.003 | 0.93 | -0.492** | -0.335* | -0.146 | -0.852**,b | 1 | ||||||||||||
| FFM | -0.845**,b | -0.003 | 0.093 | -0.492** | -0.335* | -0.146 | -0.852**,b | -0.680**,c | 1 | |||||||||||
| LBM% | -0.949** | -0.232 | -0.291* | -0.626**,c | -0.416** | -0.202 | -0.904** | -0.824**,b | -0.817**,b | 1 | ||||||||||
| WHtR | 0.615**,c | 0.465** | 0.421** | 0.986** | 0.711**,b | 0.375* | 0.625**,c | 0.706**,b | -0.515**,c | -0.598**,c | 1 | |||||||||
| BSA | 0.335* | 0.712**,b | 0.887**,b | 0.456** | 0.241* | 0.239* | 0.335* | 0.598**,c | 0.076 | -0.342* | 0.4** | 1 | ||||||||
| BAI% | 0.387** | 0.274* | 0.188 | 0.68**,c | 0.986** | -0.233 | 0.39* | 0.4* | -0.348* | -0.379* | 0.709**,b | 0.183 | 1 | |||||||
| ABSI | -0.162 | 0.03 | -0.026 | -0.116 | 0.059 | -0.183 | -0.175 | -0.131 | 0.15 | 0.274* | -0.1 | -0.04 | 0.074 | 1 | ||||||
| ABSI% | 0.597**,c | 0.063 | 0.084 | 0.799**,b | 0.635**,c | 0.182 | 0.532**,c | 0.488** | -0.562**,c | -0.604**,c | 0.790**,b | 0.1 | 0.610** | -0.22 | 1 | |||||
| ABSI-z score | 0.484** | -0.152 | -0.109 | 0.642**,c | 0.56**,c | 0.166 | 0.422** | 0.305* | -0.51**,c | -0.493** | 0.622**,c | -0.055 | 0.534**,c | -0.063 | 0.631**,c | 1 | ||||
| BMI% | 0.304* | -0.366* | -0.202 | -0.032 | 0.023 | -0.077 | 0.117 | -0.076 | -0.076 | -0.273* | -0.091 | 0.057 | -0.031 | -0.081 | 0.137 | 0.121 | 1 | |||
| BMI-z score | 0.28* | 0.794**,b | 0.871**,b | 0.405** | 0.234 | 0.157 | 0.294* | 0.623** | 0.022 | -0.291* | 0.382* | 0.753**,b | 0.205 | -0.022 | 0.085 | -0.096 | -0.216 | 1 | ||
| FMI | 0.847**,b | 0.649**,c | 0.662**,c | 0.717**,b | 0.422** | 0.332* | 0.872**,b | 1** | -0.68**,c | -0.824**,b | 0.706**,b | 0.598**,c | 0.4** | -0.131 | 0.488** | 0.305* | -0.076 | 0.623**,c | 1 | |
| FFMI | -0.845**,b | -0.003 | 0.093 | -0.492** | -0.335* | -0.146 | -0.852**,b | -0.680**,c | 1** | 0.817**,b | -0.515**,c | 0.076 | -0.348* | 0.15 | -0.562**,c | -0.510**,c | -0.076 | 0.022 | -0.68**,c | 1 |
Abbreviations: ABSI, a body shape index; BAI, body adiposity index; BMI: body mass index; BSA, body-surface area; FFM, fat free mass; FFMI, fat free mass index; FM, fat mass; FMI, fat mass index; HC, hip circumstance; LBM, lean body mass; PBF, percent body fat; WC, waist circumstance; WHR, waist to hip ratio; WHtR, waist to height ratio.
a The level of significance was * P < 0.05, and **P < 0.001.
b Moderate positive correlation.
c Low correlation.
| Arms | Trunk | Feet | |
|---|---|---|---|
| Δ FM | |||
| Slow | 0.821** | 0.643** | 0.337 |
| Rapid | 0.815** | 0.746** | 0.665** |
| Δ LBM | |||
| Slow | 0.494* | 0.429* | 0.659** |
| Rapid | 0.392* | 0.679** | 0.454** |
Abbreviations: FM, fat mass; LBM, lean body mass.
a The level of significance was *P < 0.05, **P < 0.001.
5. Discussion
The main result of our study shows that for the same total amount of WL (5% - 10% of body weight in this study), a slower WL is associated with greater reduction in WC, HC, FM/LBM, and FM in subjects with overweight and/or obesity. In contrast, RMR and LBM loss in different parts of the body were lower in slower WL group. Accordingly, the decreased levels of LBM and FFM were greater in rapid WL. On the other hand, our result showed that among all anthropometrical indices analyzed, WHtR is strongly correlated with FM during dietary WL in both groups.
Given that fat-free mass (FFM) represents a key determinant of the magnitude of RMR, a decrease in lean tissue could hinder the success of a WL program. Therefore, the loss of FM, while maintaining FFM and RMR, seems desirable (28). In agreement with our results in rapid WL group, Weinsier et al. (29), found that calorie restriction (800 kcal/d diet) caused significant decreases in RMR, which was independent of changes in body mass. The authors also found that RMR fell 6% within 10 days of energy restriction and remained 6% below baseline despite 3 - 5 month of continued energy restriction and an average 13 kg WL. Composition of meals in our study and Weinsier study were 55% vs. ~64% of the total calorie from carbohydrate, 30% vs. 14% - 20% from fat, and 15% vs. 16% - 22% from protein, respectively. Another study conducted by Biolo et al. (30), showed that the bed rest subjects lost ~2% of their LBM in hypocaloric period compared to eucaloric conditions. In this study, total energy intake was ~20% lower during the hypocaloric phases than during the correspondent eucaloric phases in bed rest. In our study, total energy intake in slow and rapid WL groups was ~26% and ~46% lower, respectively. Reduced LBM% was ~1.6 vs. ~0.2 in slow and rapid WL group, respectively. Besides, evidence has shown that visceral adipose tissue is more pathogenic than subcutaneous abdominal adipose tissue in humans, inducing systemic insulin resistance, and triggering a variety of inflammatory pathways (31, 32). Therefore, WL approaches in which visceral adipose tissue, WC and WHtR are decreased are desirable. In the present study WC and WHtR were significantly reduced in slower WL compared to rapid WL group. Chaston and Dixon (31), also reported that rapid WL demonstrated with very-low-calorie diets shows a very early but unsustained loss of visceral fat.
Studying the effects of a 4-6 weeks of very-low-calorie diet in 40 subjects with obesity, Tumova et al. (33), reported significant reduction in weight (~14%), WC and BMI. However, the effects of very-low-calorie diets on LBM, FFM, and RMR were not examined. In this study, weight reduction was induced by a protein-sparing very-low-calorie diets of approximately 800 kcal daily consisting of liquid beverages. A pilot study was also conducted by Senechal et al. (7), to compare the effects of rapid or slow WL on body composition and metabolic risk factors followed by a caloric restriction. Both groups showed significant decreases in body weight, WC and FM (total, trunk and appendicular), and similar to our study the decrease in FM (total and trunk) was found significantly greater in the slow WL group. Total LBM only decreased in the rapid WL group, which was significantly different from the slow WL group.
The results have demonstrated higher correlation between FM and WHtR among other indices such as BMI or ABSI. It seems that WHtR can be a reliable index to consider body fat alternation during weight loss strategies. Therefore, in order to evaluate FM, where the FM is not measurable, the WHtR might be the preferred index. As previously mentioned, WHtR is cheaper, easier, and more sensitive to health risk than BMI. In addition, the cutoff point of 0.5 for both sexes has been suggested. WHtR may allow the same boundary values for both children and adults (18).
Based on the result of our study, WHtR is not only an appropriate index of obesity, however, its fluctuation during WL is also highly correlated with FM changes (more than other indices). To the best of our knowledge, this is the first study showing a strong positive association between WHtR and FM during a dietary WL intervention with different rates. The findings of this study underlined that during WL, WHtR appears to be a potent index to monitor FM reduction and can replace BMI as an indicator of obesity and to monitor FM fluctuations in clinical nutrition.
In addition to WHtR, there are some indices like ABSI and BAI that can be valuable during weight loss. In the present study there were no significant differences between two weight loss groups in regards to the amount of weight loss, however, ABSI significantly decreased in the slow WL group. The newly developed and applied ABSI is based on WC, weight and height, where high ABSI indicates that WC is higher than expected for a given height and weight and corresponds to a more central body size (15). Applying ABSI along with BMI as a predictor variable separates the influence of the components of body shape measured by WC from that of body size. Krakauer and Krakauer (15), also mentioned that at a given height and weight, high ABSI may be correlated with a greater fraction of visceral (abdominal) fat compared to peripheral tissue. They also reported that body shape, as measured by ABSI, had little correlation with height, weight, or BMI (15). In the present study ABSI only had a low correlation with LBM% without any correlation with other indices. However, ABSI% was highly correlated with WC (r = 0.779) and WHtR (r = 0.790). After considering age and sex for ABSI and calculating ABSI z score, the correlation was observed between ABSI z score and PBF, WC, HC, FM/LBM, FM, FFM, LBM%, WHtR, BAI%, and ABSI%. Overall, the results of the present study recommend to match the age and sex for ABSI. Besides, we suggest that the ABSI and related measures to be evaluated in different ranges of BMI to ensure the utility of ABSI’s.
To our result, there was a strong correlation between PBF with LBM% and FM/LBM, and a high correlation between PBF and FM, FMI and FFMI. However, the observed significant correlation between PBF and other variables, such as WC, HC WHtR, ABSI etc. were moderate. In line with our study (r = 0.426), the Bergman et al. (16), study, found that hip circumference is correlated with PBF (r = 0.602). In addition, there was significant correlation between BAI% and PBF in both studies. Bergman et al. (16), suggested that BAI can be used in the clinical settings even in remote locations with very limited access to reliable scales. However, since the observed correlation was weaker in our study, this mentioned suggestion cannot be confirmed. It is worth mentioning that in the Bergman study the precent of body fat was measured by the dual-energy X-ray absorptiometry, thus, the different results might be due to different measuring tools.
As our results showed, the most reduction of FM was related to arms in both slow and rapid WL groups. The reduction of FM in feet was higher in rapid WL groups compared to slow WL. It might be concluded that rapid WL might be a better option to help those who want to lose more FM in feet. Furthermore, reduction in trunk FM was higher in rapid WL group. Evidence has consistently reported that increased amount of abdominal adipose tissue is strongly related to cardiovascular diseases risk factors, as well as to increased morbidity and overall mortality (34). Therefore, it might be concluded that rapid WL is more useful in order to attenuate cardiovascular diseases risk factors. The capability of maintaining the reduced amount in long-term periods, however, needed further considerations. Accordingly, in order to make reasonable recommendations we suggest other studies to be designed to evaluate the maintenance of reduced FM in long term periods.
5.1. Conclusions
Our findings indicate that FM reduction in rapid WL was more than slow WL, however, reduction in FFM, RMR, LBM, LBM% was also higher in this group. In contrast, WC, HC, and FM/LBM reductions were much higher in the slow WL group. In addition, high correlation was observed between FM and WHtR, among other indices. In other words, our study presents a new potential clinical application of WHtR as a highly-correlated index with FM during WL regardless of the rate of WL. Moreover, long-term WL program may prevent inevitable loss of LBM, which makes it as an appropriate WL strategy in clinical settings. To monitor the WL and body fat changes in WL programs we propose FM, WHtR, and FM/LBM to be assessed as a complementary tool beside BMI, weight and WC.
Many studies have suggested that rapid weight loss may serve as a risk factor for later weight regain. In addition, many studies are using dual energy X-ray absorptiometry (DXA) for analyzing the body composition. Thus, a limitation of the current study was that it did not evaluate weight regain and did not use DXA.