1. Context
Hemostasis is an essential process that maintains the integrity of the blood stream in the human body. This process is achieved through a balance between hemostatic plug formation (primary hemostasis) and thrombin generation (secondary hemostasis) in one hand, and fibrinolytic system, on the other hand, to maintain vascular patency (1-6). Disequilibrium between activators and inhibitors of the hemostatic system may result in bleeding or pathological thrombosis. Tendency to thrombosis, arterial and/or venous, is associated with increased morbidity and mortality (1, 2, 7).
Thyroid hormones are potent mediators of numerous physiological processes and their abnormalities can adversely affect various steps in the coagulation cascade (8-11). The earliest reports on a link between thyroid disorders and coagulation abnormalities are from the early years of the past century (12-14). Thyroid dysfunction has been associated with contradictory findings on coagulation abnormalities, ranging from subclinical laboratory derangements to clinical bleeding and thromboembolism (15-25). The present report provides a review on hemostatic alteration in hyperthyroidism.
2. Methods
A comprehensive review of the literature has been done and the following search terms (medical subject heading, terms, and text words) were used for the MEDLINE search until March 2016: Hyperthyroidism; thyrotoxicosis; Graves disease; goiter, nodular; hemostasis; thyroid hormones; blood coagulation factors; blood coagulation tests; blood coagulation factors; blood coagulation disorders; venous thromboembolism; bleeding; fibrinolysis; receptors thyroid hormone; hemostasis; thyroiditis, autoimmune. In addition, by hand-searching reference lists in review articles and relevant textbook chapters were searched for papers beyond 2000. The eligibility criteria were inclusion of all types of studies that evaluated alterations in hemostatic parameters and/or occurrence of clinical hemostatic events including venous thromboembolism (VTE) and/or bleeding in any type of hyperthyroidism. The authors reviewed all the titles and abstracts generated by the search engine and exclude those that were not covering the eligibility criteria. For potentially relevant studies hard copies of the full article was obtained and the studies were reviewed in detail to make sure they met the inclusion criteria. Figure 1 shows the flowchart of studies assessed and selected by two reviewers. A total of 878 papers were cited and after exclusion 637 papers that did not meet inclusion criteria, 241 papers were thoroughly studied and in order to submit the current manuscript to this journal, the number of papers was further decreased (Figure 1). In order to prevent missing any scientific points from the omitted papers, review articles containing those scientific points were used in the manuscript’s reference list. Throughout the manuscript, evidence in favor of hypercoagulability, hypocoagulability, or no alterations in hemostatic parameters, if applicable, has been provided.
3. Results
3.1. Overt Hyperthyroidism
Hyperthyroidism is found in 1.3% of the U.S. population of which 0.5% is clinical- and 0.7% subclinical hyperthyroidism (26)
3.1.1. Hypercoagulability
The initial reports regarding thyroid and coagulation disorders were in 1913 and 1927 reporting an association between thyrotoxicosis and cerebral venous thrombosis (12-14). Several case reports described individuals with hyperthyroidism and cerebral venous thrombosis (27-31). Subsequent studies evaluated thyroid dysfunctions in relationship to alterations in coagulation-fibrinolytic system. Most reports supported a hypercoagulable state and hypofibrinolysis in hyperthyroidism (32-34).
Patients with hyperthyroidism display a tendency to develop thromboembolic complications, with major embolism accounting for up to 18% of deaths in patients dying from thyrotoxicosis (35, 36). The incidence of arterial thromboembolism in patients with atrial fibrillation is higher in those with hyperthyroidism than non-thyrotoxic individuals (37).
Hyperthyroidism and an excess of thyroid hormones are associated with a hypercoagulable state (9, 32) and these has led to the hypothesis that hyperthyroidism maybe considered a risk factor for venous thromboembolism. Confirmation of this association may change clinical practice as for decision whether venous thromboembolism (VTE) in a hyperthyroid patient would be considered provoked or unprovoked. Some authors assert that hyperthyroidism as a risk for VTE (38).
Increased and reduced risks of VTE have been associated with high and low levels of free T4, respectively (39). In a recent case report, the authors presented two patients with severe VTE with hyperthyroidism and no other risk factor for VTE, emphasizing possible association between hyperthyroidism and VTE (40). However, studies on the effect of elevated free T4 on the occurrence of VTE are scarce and there has been controversy over whether thyroid hormone excess leads to an increased risk of thrombosis (41-43). Recent large-scale studies have shown an association between VTE and high free T4 levels. Following age- and sex-adjustment, Free T4 levels above 17 pmol/L resulted in an odds ratio (OR) and 95 % confidence interval (95% CI) of 2.2 (95% CI, 1.2 - 4.2) for DVT. This further increased up to an OR of 13.0 (95% confidence interval (CI), 1.1 - 154.1) for free T4 levels above reference range. The authors suggest increasing levels of free T4 to be a risk factor for VTE (39). The HUNT2 cohort study in Norway, showed that there is a ~10 fold increased risk of occurrence of VTE for free T4 levels > 17.3 pmol/L (i.e., above 98th percentile) after adjustment for age, sex, and body mass index. The authors concluded that free T4 levels at higher end of normal range are strong risk factor for VTE (44). In a nation-wide longitudinal study for 5 years including 8,903 individuals with hyperthyroidism as a study cohort and 44,515 individuals without hyperthyroidism as the comparison controls, there was a 2.31 times greater risk (95% CI, 1.20 - 4.45, P = 0.012) of pulmonary embolism (PE) in patients with hyperthyroidism than in the comparison cohort (45). In the MEGA study, which is a large population-based case-control study, 2,177 individuals with DVT and/or PE were recruited as cases as well as 2,826 age- and sex-matched individuals as controls. Increases in mean levels of FVIII, FIX, fibrinogen, and vWF were observed with increasing levels of free T4 among the controls, which showed that high free T4 values were associated with increased levels of prothrombotic factors. Additionally, there was an association between free T4 levels and both DVT and PE. For levels of free T4 > 24.4, pmol/L, the OR (95% CI) for DVT was 2.7 (95% CI, 1.2 - 6.1) and for levels of free T4 of 22.2 pmol/L, the OR (95% CI) for PE was 2.5 (1.2 - 5.1). It was concluded that high free T4 values increase the risk of VTE by increasing the concentration of circulating FVIII, FIX, fibrinogen, and vWF (46). A retrospective cohort study including 587 hyperthyroid patients and diagnosis of VTE within 6 months before or after diagnosis of hyperthyroidism was conducted in the Netherlands. The study revealed an incidence rate of 8.7 (95% CI 2.8 - 20.2) per 1,000 person-years. Three of these five patients had a first VTE, giving an incidence rate of 5.3 (95% CI 1.1 - 15.6) per 1,000 person-years for first VTE. In the general population, the incidence rate for first VTE is between 0.6 - 1.6 and for all VTE is between 0.7 - 1.8 per 1,000 person-years. Therefore, the incidence of VTE in patients with hyperthyroidism is higher than the general population (47). In a large-scale retrospective cohort study, databases of linked statistical records of hospital admissions and death certificates for the Oxford record linkage study area (ORLS1:1968 to 1998 and ORLS2:1999 to 2008) and the whole of England (1999 to 2008) were analyzed. Rate ratios for VTE were determined, comparing immune-mediated disease cohorts with comparison cohorts. Significantly but mildly elevated risk for VTE was found in thyrotoxicosis in ORLS2 population with a rate ratio of 1.56 (95%CI, 1.23 - 1.95, P < 0.001) and in the England data set with a rate ratio of 1.34 (95%CI, 1.27 - 1.42, P < 0.001), but not in ORLS1 population. Systemic lupus erythematous showed the highest risk ratio among several immune-mediated disorders in the study (48).
Qualitative and quantitative platelet abnormalities have been reported in patients with hyperthyroidism (49-53); however, there are studies where no significant change in platelet count has been observed in hyperthyroid patients compared to controls (50). A study on parameters of primary hemostasis in both hypo- and hyperthyroid patients showed that hyperthyroid patients had normal bleeding time, although agglutination induced by ristocetin as marginally reduced. Template bleeding time was significantly increased during treatment but remained within normal range and no significant change occurred in platelet agglutination or aggregation due to treatment (51).
The large cross sectional Study of Health in Pomerania in Germany showed higher plasma fibrinogen in overt hyperthyroidism (mean TSH of 0.04 mU/L and elevated free T4) compared to euthyroids (mean TSH of 0.82 and normal free T4). This is in support of a procoagulant effect in hyperthyroidism (54).
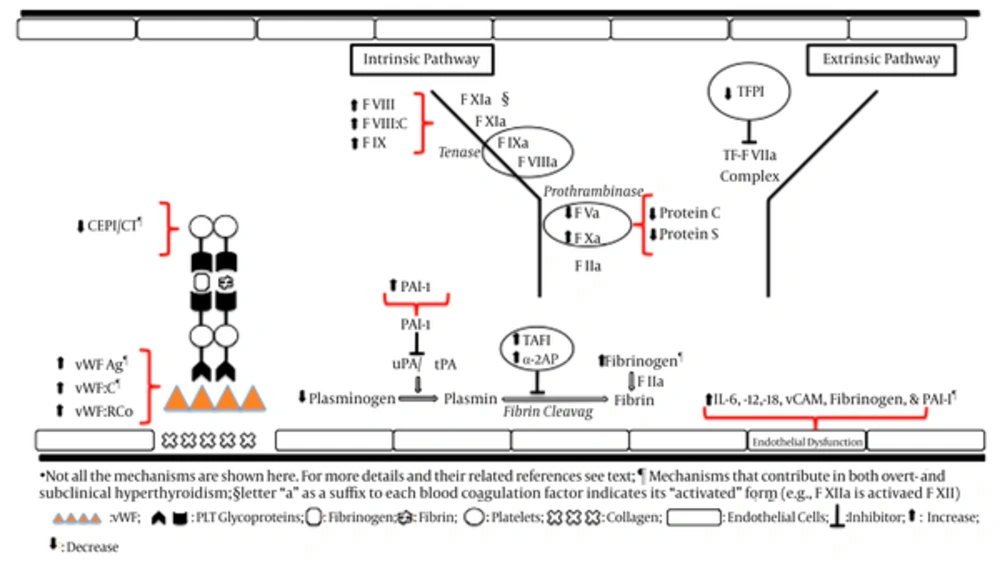
Significant decrease in PT and aPTT and elevation in levels of fibrinogen have been observed in hyperthyroidism, which is in favor of a hypercoagulable state (50). Shortened aPTT was observed in hyperthyroidism in a study on a general population of unselected outpatients (55). Hyperthyroidism renders a hypercoagulable and hypofibrinolysis states (32, 34, 49) and is associated with significantly increased fibrinogen (that may promote hypercoagulability), decreased plasminogen and plasminogen activator, and non-significant changes in PAI and antiplasmin levels (56), increased levels of FVIII, vWF, and fibrinogen (18, 19, 32, 57), increased vWF activity and fibrinogen levels (18, 58, 59), and decreased t-PA levels (18). Elevated plasma levels of FVIII, FIX, vWF, and fibrinogen and increased PAI-1 (that decreases fibrinolysis) have been reported in hyperthyroidism and in healthy individual taking thyroid hormones (18, 32, 54, 55, 60-62). Increased plasma levels of fibrinogen and fibrinopeptide Bβ 15 - 42, a specific product of fibrinogen metabolism induced by plasmin, were observed in hyperthyroid patients. The restoration of euthyroidism either by antithyroid drug or by radioiodine caused a significant decrease of fibrinogen and β beta 15 - 42 (22). However, the majority of these studies methodological limitations and deduction of a net effect of excess thyroid hormone on heamostatic system remains to be clarified (32). Using medium quality observational studies in a meta-analysis revealed significant decrease in bleeding time and plasminogen, increase in α-2 antiplasmin (and therefore, a possible increase in inactivation of plasmin), no difference in prothrombin fragment 1 - 2 and ristocetin agglutination and tissue plasminogen activator activity and plasmin-antiplasmin complex. Levels of vWF antigen, fibrinogen, tissue plasminogen activator antigen (tPA:Ag), and plasminogen activator inhibitor 1 (PAI-1) were reported as increased or unchanged in different studies (32).
Hyperthyroidism-induced vWF elevation is associated with enhanced platelet function and therefore shortened collagen-epinephrine-induced closure time (CEPI-CT) values. These changes may contribute to the higher risk for cardiovascular disease in patients with hyperthyroidism (63). Platelet plug formation decreases during therapy with thiamazole (57).
In a case-control study, including 42 Graves’ disease and 75 toxic nodular goiter as the hyperthyroid group versus 39 healthy controls, circulating markers of endothelial dysfunction, including interleukin (IL) -6, IL-12, IL-18, fibrinogen, plasminogen activator inhibitor 1 (PAI-1), vWF, and vascular cell adhesion molecule-1 (sVCAM-1) were significantly elevated in the patients with both overt hyperthyroidism and subclinical hyperthyroidism. The results suggest that both overt- and subclinical hyperthyroidism may be associated with endothelial dysfunction, which is reflected by decreased fibrinolytic activity, hypercoagulability, and increased levels of IL-6, IL-12, and IL-18 and depends not only on the cause but also on the degree of hyperthyroidism (64). Plasma PAI-1 activity, but not adipose tissue secretion of PAI-1, was increased in hyperthyroid Graves’ disease before treatment as compared to during anti-thyroid treatment (P = 0.01) and to euthyroid controls (P = 0.0001). Hyperthyroidism is associated with the formation of a compact fibrin network ex vivo, which are resistant to fibrinolysis (65), representing one plausible mechanism explaining increased risk of thrombosis in hyperthyroid patients (66). Secretion of IL-6 by adipose tissue was increased in hyperthyroid state both before and during anti-thyroid treatment compared to controls, which suggests thyroid autoimmune pathology may regulate secretion of IL-6 from adipose tissue (67).
In hyperthyroid patients the plasma concentrations of AVP and endothelium-associated proteins (EAP) were significantly higher than in the control group. Rendering hyperthyroid patients into the euthyroid state significantly lowered AVP, fibronectin, and vWF compared with pretreatment levels (61).
Hyperthyroid patients with Graves’ disease showed significantly elevated levels of plasma thrombomodulin and vWF antigen, which both are synthesized by endothelial cells and are known as markers of endothelial injury. By normalization of thyroid function, the increased levels returned to normal (58, 68, 69).
Iatrogenic hyperthyroidism in a patient with extremely high single dose (25 mg) of levothyroxine ingestion following a suicidal attempt showed increased levels of FVIII, FIX, FX, von Willebrand factor ristocetin cofactor activity (VWF:RiCo), von Willebrand factor antigen (vWF:Ag), plasminogen activator inhibitor 1 (PAI-1), and endogenous thrombin potential. No clear change was observed in FII, FVII, PT, aPTT, protein C activity, total and free protein S antigen, and prothrombin fragment. The findings suggest that thyroid hormone excess shifts the hemostasis balance towards a hypercoagulable and hypofibrinolysis state, increasing the risk of VTE (70). T3 upregulated coagulation factor genes resulting in T3-induction ratios of 8-fold for thrombin, 4.9-fold for FX, and 2- to 3-fold for fibrinogen transcription (11).
Both elevated and low TAFI antigen levels have been reported for hyperthyroid patients (71, 72). Elevated TAFI has been detected in several studies on hyperthyroidism. Increased TAFI and decreased FV, protein C, protein S, and tissue factor pathway inhibitor (TFPI) in hyperthyroid patients represent a potential hypercoagulable and hypofibrinolytic state, which might augment the risk for atherosclerotic and atherothrombotic complications (72). The changes in hemostatic parameters in favor of a hypercoagulable state in overt hyperthyroidism are summarized in Table 1.
| Changes in Hemostatic Parameters | References |
|---|---|
| Increased level of fibrinogen, FVIII, FIX, FX, vWF & vWF Ag, PAI-1 and vWF:C, FVIII:C, vWF:RCo | (18, 19, 32, 46, 50, 54-62, 64, 70) |
| Increased IL-6, IL-12, IL-18, and sVCAM-1 and fibrinogen and PAI-1 (indicating endothelial dysfunction predisposing a decreased fibrinolytic activity and hypercoagulability) | |
| Increased fibrinopeptide Bβ 15 - 42 and fibrinopeptide A | (22, 32) |
| Increased plasma thrombomodulin | (57, 68, 69) |
| Increased α2-antiplasmin | (32) |
| Increased TAFI | (72) |
| Increased formation of compact fibrin ex vivo resistant to fibrinolysis | (65) |
| Decrease in CEPI/CT reflecting enhanced PLT function | |
| Decrease in FV and protein C and protein S and TFPI | (72) |
| Decrease in PT and aPTT | (50) |
| Decreased plasminogen | (32, 56) |
| Decreased plasminogen activator [t-PA] | (56) |
Abbreviations: Ag, antigen; aPTT, activated thromboplastin time; C, activity; CEPI/CT, closure time with collagen/epinephrine; F, factor; IL, interleukin; PAI-1, plasminogen activator inhibitor 1; PT, Prothrombin time; t-PA, tissue-type plasminogen activator; TAFI, thrombin activatable fibrinolysis inhibitor; TFPI, tissue factor pathway inhibitor; u-PA, urokinase; vW, von Willebrand; vWF:RCo, von Willebrand ristocetin cofactor; sVCAM-1 soluble vascular cell adhesion molcule-1.
aSome authors consider elevation antithrombin III as a change in favor of a prothrombotic effect (18)
3.1.2. Hypocoagulability or No Alterations in Hemostasis
There are some studies that are inconsistent with enhanced hypercoagulability in hyperthyroidism. In a large-scale study from the national hospital discharge survey (NHDS) databank for the patients being discharged from the hospital, hyperthyroidism was not associated with an increased risk of VTE (relative risk = 0.98, 95% CI = 0.96 - 1.01) (43). A recent meta-analysis has shown under-reporting of thromboembolic events in clinical trials (73). Although the study on lack of association of hyperthyroidism and VTE is not a meta-analysis (43), however, there is a potential that this under-reporting would also be present among their VTE cases (43), and therefore, become the source of error in the results and their interpretation. Limitations related to VTE identification and classification, the underlying cancer, and to management process, such as inadequate documentation may result in this underestimation (74).
Development of Graves’ disease in a patient with chronic Hashimoto’s thyroiditis was accompanied with a hemorrhagic disorder with bleeding into muscle, joints, and skin, secondary to an acquired FVIII deficiency due to a factor VIII inhibitor (75). A severe hemorrhagic disorder due to factor VIIIc antibody, in a patient with hyperthyroid Graves’ disease not associated to overt clinical features was reported (76).
In a study, half of Graves’ disease individuals, however, had low platelet count and shortened platelet survival that returned to normal levels after antithyroid treatment (52). The platelet changes observed in hyperthyroidism, such as lower platelet count and increased mean platelet volume in conjunction with a shortened platelet lifespan reflect metabolically rather than immunologically mediated phenomena (53).
On the contrary to abovementioned reports (72), low TAFI antigen levels have been reported in overt hyperthyroidism (71). Apparently, this is in favor of a hypocoagulable state as TAFI attenuates fibrinolysis. However, authors found PAI-1 was elevated as well. The inverse correlation of two inhibitors of fibrinolytic system is a complex condition, and therefore, the authors assumed that this low TAFI antigen level might be due to activation of TAFI pathway (71). Levothyroxine suppression therapy in premenopausal women with benign thyroid nodules resulted in non-significant decrease in TAFI antigen and increase in PAI-1 antigen levels (77).
Hyperthyroidism has increased metabolic clearance of FII, FVIII, FIX, and FX and lowered activity of FII (78). Decreased levels of FX (18) and increased levels of antithrombin III have been reported earlier, although the latter has not been attributed to a bleeding tendency (18). Warfarin produced a greater fall in FII and FVII and a greater increase in prothrombin ratio and partial thromboplastin time with kaolin in the hyperthyroid state than in the euthyroid state (79, 80).
Plasminogen activators of tissue- (t-PA) and urokinase- (u-PA) type are known to be potent stimulators of plasmin formation in blood plasma. The fibrinolytic system is activated when t-PA transforms plasminogen to plasmin. Plasmin is a very strong proteolytic enzyme, which can digest not only fibrin if it is present in the vessel system but also other proteins such as coagulation factors. Only free t-PA has fibrinolytic activity and is inhibited when PAI-1 and other inhibitors form an enzymatically inactive complex with t-PA. PAI, such as PAI-1, is a strong inhibitor of fibrinolytic activation (81). Plasma concentrations of t-PA, u-PA and PAI-1 were significantly higher in 33 hyperthyroid Grave’s cases than 33 controls. Interpretation of the results is difficult and the authors assumed that prothrombotic effect of PAI-1 elevation was in response to the hypocoagulation effect of elevated t-PA and u-PA (82).
Tissue factor pathway inhibitor (TFPI) is a vascular anticoagulant, which inhibits the initial reaction of the tissue factor-mediated coagulation pathway. It dampens the initiation of blood coagulation by inhibiting two potent procoagulant complexes, tissue factor-factor VIIa (TF-FVIIa) and early forms of prothrombinase. Alternative splicing of mRNA produces two TFPI isoforms, TFPIα and TFPIβ. Both isoforms inhibit TF-FVIIa, but only TFPIα can inhibit early forms of prothrombinase. TFPIα and TFPIβ are produced in cultured human endothelial cells. Some portion is constantly released into plasma as free form and some binds to lipoprotein particles. Pharmaceutical agents have been developed that block TFPI function to treat hemophilia (83). In a study of 15 patients with hyperthyroidism and Graves’ disease, 10 patients with anti-thyroid medication-induced euthyroidism and Graves’ disease, and 25 controls, plasma levels of total TFPI and free TFPI were significantly higher in hyperthyroid Grave’s patients compared to euthyroid Graves’ and controls. This appears to be in favor of a hypocoagulation state. Free TFPI was similar between Euthyroid Grave’s and controls. Plasma free TFPI levels correlates closely with free T3 levels, which suggests that thyroid hormones might influence synthesis and metabolism of TFPI. No relationship was found between plasma free TFPI and thyroid autoantibodies and therefore, it was assumed that autoimmune component of Graves’ disease had no effect on plasma free TFPI levels (68).
Hyperthyroidism has been reported to be associated with no change from normal in clotting factors II, V, IX, and X (84).
For hyperthyroidism and its effect on coagulation system proposed mechanisms are, but not limited to, direct and indirect effects of thyroid hormone excess and autoimmunity. In a study on 35 non-smoking, postmenopausal women, aged 51 - 69, with subclinical or overt hyperthyroidism elevated fibrinogen and D-dimers levels were observed in hyperthyroid subjects. There was a negative correlation between plasma fibrinogen and D-dimers levels and anti-thyroid perioxidase antibodies (TPOAb) levels. The changes in fibrinogen and D-dimer levels were reversible as they decreased after radioiodine therapy. The authors assumed that this reversal might relate to the severity of hyperfunctioning and autoimmunity of thyroid disorder. The findings are supportive for hypercoagulation and hyperfibrinolysis states (85). In summary, several changes in coagulation factors are supportive of a prothrombotic state in subclinical hyperthyroidism (18, 32, 34, 49, 54, 57, 59, 86) and are summarized in Table 2.
| Changes in Hemostatic Parameters | References |
|---|---|
| Increased FVIII inhibitor causing acquired FVIII deficiency | (75, 76) |
| Increased metabolic clearance of FII, FVIII, FIX, FX | (78) |
| Increased plasma t-PA and u-PA | (82) |
| Increased total and free TFPI | (68) |
| Increased mean PLT volume | (53) |
| Decreased PLT count | (49, 52, 53) |
| Decreased (shortened) PLT survival (life span) that returned to normal levels after antithyroid treatment | (49, 52, 53) |
| Decreased TAFI Ag | (71) |
| Decreased FII:C | (78) |
| Decreased FX | (18) |
Abbreviations: Ag, antigen; :C, activity; F, factor; PLT, platelet; t-PA, tissue-type plasminogen activator; TFPI, tissue factor pathway inhibitor; TAFI, thrombin activatable fibrinolysis inhibitor; u-PA, urokinase.
3.2. Subclinical Hyperthyroidism
National health and nutrition examination survey (NHANES III) showed subclinical hyperthyroidism in 0.7% of the U.S. population (26).
3.2.1. Hypercoagulability
Subclinical hyperthyroidism, which is defined as low TSH and normal freeT4 and T3 is typically caused by the similar conditions that result in overt hyperthyroidism. The clinical implications of subclinical hyperthyroidism have been reviewed elsewhere (87). Undesirable effects of the subclinical hyperthyroidism on cardiovascular system are not different whether its etiology is endogenous or exogenous (88). However, the association between subclinical hyperthyroidism and cardiovascular morbidity and mortality is controversial (89-92). Studies on subclinical hyperthyroidism and hemostasis-fibrinolytic system are scarce (10, 93).
A large cross sectional Study of Health in Pomerania in Germany showed higher plasma fibrinogen in subclinical hyperthyroidism (mean TSH of 0.2 mU/L and normal free T4) versus euthyroids (mean TSH of 0.82 and normal free T4). This is in favor of a potentially prothrombotic state. TSH was an independent risk factor for elevated levels of plasma fibrinogen (54).
A meta-analysis on observational studies with medium-quality scores revealed significantly increased vWF:C and fibrinogen levels. A hypercoagulable state is considered to be present in subclinical hyperthyroidism (32, 49). Increased factor X activity in patients with subclinical hyperthyroidism represents a potential hypercoagulable state, which might augment the already existing risk for atheroscleroic complications (86). Numerous clinical and experimental studies suggest that elevated vWF levels reflect endothelial dysfunction or damage. A study on 20 subclinical hyperthyroid versus 20 euthyroid subjects showed significantly higher levels of vWF in subclinical hyperthyroid individuals (94). This could contribute to an increase in the risk of cardiovascular events in subclinical hyperthyroidism.
A study on iatrogenic (thyroidectomy- and radioiodine-induced) hypothyroidism compared to iatrogenic (thyroxine-induced) subclinical hyperthyroidism on the same patients revealed that vWF and fibrinogen were significantly higher in the latter, though vWF was still within normal range and fibrinogen exceeded slightly above the normal limit (25). This is in support of a tendency toward a prothrombotic status in subclinical hyperthyroidism (95). Another study was conducted on 90 thyroidectomized patients (due to differentiated thyroid cancer), followed by radioactive iodine treatment for remnant ablation, was conducted. Multiple hemostatic parameters in severe hypothyroidism versus subclinical (mild) hyperthyroidism (suppressed TSH and normal free T4 and free T3) were checked before and after levothyroxine suppression treatment. To prevent any effect of residual disease, those with measurable serum thyroglobuin or anti-thyroglobulin antobodies were excluded. Subclinical hyperthyroidism was associated with a hypercoagulable/hypofibrinolytic state and alteration of primary hemostasis toward prothrombotic state. These were reflected by a significant increased in plasma levels of PAI-1, FVIII, vWF antigen, fibrinogen, and antithrombin and a significant reduction in closure time with collagen/epinephrine (CEPI-CT), in closure time with collagen/ADP in platelet function analyzer (PFA-100), a levels of prothrombin fragment 1 + 2. Tissue plasminogen activator antigen was similar between two groups (63).
Subclinical hyperthyroidism was associated with significantly increased interleukin (IL) -6, IL-12, plasminogen activator inhibitor 1 (PAI-1), and vascular cell adhesion molecule-1 (sVCAM-1) compared to controls. However, levels of fibrinogen, vWF, and IL-18 were similar. The authors assumed that the results may suggest that subclinical hyperthyroidism may be associated with endothelial dysfunction, which is reflected by decreased fibrinolytic activity, hypercoagulability, and increased levels of IL-6, IL-12, and depends not only on the cause but also on the degree of hyperthyroidism, as overt hyperthyroid patients showed higher levels of markers of endothelial dysfunction compared to the subclinical hyperthyroid cases (64).
Elevated fibrinogen is an independent risk factor for cerebrovascular disorders and elevated D-dimer levels have been associated them as well. In 36 subclinical hyperthyroid- and 36 euthyroid control subjects matched for age, gender, and body mass index, fibrinogen and D-dimer levels were significantly higher in the subclinical hyperthyroidic- than in the euthyroid group, suggesting a relatively hypercoagulable state in subclinical hyperthyroidism (96). The changes in hemostatic parameters in favor of a hypercoagulable state in subclinical hyperthyroidism are summarized in Table 3.
| Changes in Hemostatic Parameters | References |
|---|---|
| Hypercoagulability | |
| Increased level of fibrinogen | (25, 32, 54, 63, 96) |
| Increased vWF:C | (32, 49) |
| Increased FX:C | (86) |
| Increased level of vWF (vWF Ag) and FVIII | (25, 63, 94, 95) |
| Increased IL-6, IL-12, PAI-1, and sVCAM-1 reflecting endothelial dysfunction rendering a hypercoagulable state | (64) |
| Decrease in closure time with collagen/epinephrine (CEPI/CT) and Collagen/ADP in pletelet function analyzer [PFA-100], reflecting enhanced PLT function Hypocoagulability | (63) |
| Decreased TAFI Ag | (71) |
Abbreviations: ADP = adenosine diphosphate; Ag = antigen; :C = activity; F = factor; IL = interleukin; PAI-1 = plasminogen activator inhibitor 1; sVCAM-1 = soluble vascular cell adhesion molcule-1; vW= von Willebrand.
aElevation antithrombin has been considered as a change in favor of a prothrombotic effect (18, 63).
3.2.2. Hypocoagulability or No Alteration in Hemostasis
Subclinical hyperthyroid patients showed lower TAFI antigen levels compared to the controls, which is in favor of hypocoagulation. Although the TAFI levels were not quite statistically significant (P = 0.508), their levels were similar to overt hyperthyroidism and, additionally, there were low number of cases assessed in the study. On the other hand, PAI-1 levels were elevated as well. As both PAI-1 and TAFI are inhibitors of fibrinolytic system, a reverse correlation between two inhibitors is a complex situation. The authors assume that this might be a reflection of an activation of TAFI pathway (71). In a study in Sudan, PT, aPTT, fibrinogen, and platelet counts were not different in subclinical- and overt hyperthyroidism (50). In general, the studies on subclinical hyperthyroidism and hemostasis are scarce and current findings are more in favor of a hypercoagulable state. More studies are required in this regard (see Table 3).
Abbrevitions: Ag, antigen; AIT, autoimmune thyroid disorders; F, coagulation factor; FVIII:C, FVIII activity; FVII:C, FVII activity; FVII activity; FVIIa, activated FVII; PAI-1, plasminogen activator inhibitor 1; PLT, platelets; SCH, subclinical hypothyroidism; tPA, tissue plasminogen activator; TF, tissue factor; Vwf, von Willebrand factor; vWF:C, vWF activity; uPA, Urokinase type plasminogen activator; α2 - AP = α2.
4. Conclusions
Although most of the studies are in favor a hypercoagulable state in both overt- and subclinical hyperthyroidism, however, this relationship is mostly underappreciated as it may be partly due to the fact that the majority of the studies have not been associated with relevant clinical implications. There are an increasing number of population-based studies in favor of hyperthyroidism as a risk factor for VTE. However, there is still a need for large-scale, prospective, multicenter studies to confirm it. Of course, finding a control group with overt hyperthyroidism who would serve as the control group for a prospective study for evaluation of VTE and/or bleeding occurrence is almost practically not feasible, and instead the general population may be recruited instead, however, this would not be that much an issue with subclinical hyperthyroidism. The number of studies on subclinical hyperthyroidism is scarce though. Confirmation that overt- and/or subclinical hyperthyroidism would be a risk factor for VTE, and therefore a patient with VTE and hyperthyroidism can be classified as “provoked” or “unprovoked” has a significant impact on clinical management of the patients and the duration of anticoagulation therapy.