1. Introduction
Physicians have long dedicated themselves to the promotion of health and prevention of disease in mankind. Rabi-Ebn-Ahmad Akhvaini, a Persian scholar, defined medicine as a profession that maintains health status, when disturbed by disease and restores health in man, using knowledge and practice (1). Preventive medicine is, hence, concerned with precluding the appearance of risk factors, delaying the progression to overt disease, and decreasing the impact of illness. By definition, there are 4 distinct types of prevention in different phases of disease (2). Primordial prevention is the maintenance and promotion of health in the community by averting the appearance of risk factors of a disease. Primary prevention is performed by controlling or reducing risk factors to prevent overt disease in previously healthy individuals. Secondary prevention is avoiding the progression of latent or mild illness to more advanced stages (presence of physiological abnormality without sign or symptom manifestation). Tertiary prevention is the optimization of medical care and management to improve an already established disease and avoidance of complications and disabilities (presence of sign and symptoms) (3). Quaternary prevention is a newer concept that refers to actions taken to identify a patient or a population at risk of over-medicalisation to protect them from invasive medical interventions and provide them with care procedures that are ethically acceptable. Approaches to prevention overlap and merge, yet all levels are equally important and complementary. Primordial and primary preventions are related to health of the whole population, while secondary and tertiary preventions are generally focused on people who already have signs of disease.
During the past decades, much success has been achieved in controlling several infectious and non-communicable diseases in many high-income countries, and some developing countries (4), however, there is limited data of comprehensively designed studies on prevention of thyroid disorders.
In the realm of thyroid disease, prevention of iodine deficiency disorders (IDD) by adequate iodine nutrition has been recognized and implemented in many parts of the world in the past century (5); this is a classic form of primordial prevention for health promotion and avoidance of iodine deficiency, a known risk factor for goiter, hypothyroidism, mental deficiency, and other manifestations of iodine deficiency disorders (IDD). Preventing common environmental exposures such as perchlorate, thiocyanate, and nitrate are other primordial preventive measures against thyroid disorders; however, evidence for their established role in causing disease is unclear. Understanding and identifying potential risk factors of thyroid autoimmunity to prevent its occurrence is another type of primordial prevention.
Smoking cessation, control of alcohol consumption, and thyroid autoimmunity (positive antibody alone) are listed among measures of primary prevention of thyroid disorders.
Therefore, the main question is “What are the effective factors contributing to primordial and primary prevention of thyroid diseases?” Except for prevention of IDD, articles dealing with other aspects of prevention in thyroid disease are scanty (6). Therefore, this research aimed at reviewing the data available on primordial and primary preventions of thyroid disease.
2. Methods
The terms "prevention" OR "screening" with either of the following keywords, “iodine”, “iodine deficiency”, “thyroid”, “hypothyroidism”, “hyperthyroidism”, “thyroid nodules”, and “thyroid cancer” were used to search Medline for papers published from July 2001 to July 2015. A combination of more general search term usage with additional filtering of articles was applied to include all relevant articles related to the 4 types of prevention in thyroid disease. After reviewing, authors divided the articles into 2 parts: one related to primary and primordial (446 articles) and the other related to secondary and tertiary preventions (2361 articles). We reviewed all abstracts of studies published in both French and English and included those appropriately designed. Two librarians at our institute assisted us in searching for articles. An article had to meet the following criteria to be qualified:
a) Having an appropriate study design of clinical trials, cohort, case control studies, or surveys.
b) Being a scholarly written review article.
c) Having followed guidelines of major international organizations.
d) Making the reviewer aware of authors’ names; this was done deliberately to allow the reviewer to include all pertinent studies of initial 446 articles related to primary and primordial preventions; 86 articles fulfilled the above-mentioned criteria and were selected for this review, and their results were included in pre-designed tables, from which the data used in this review were extracted.
2.1. Primordial Prevention of Thyroid Disease
2.1.1. Iodine Nutrition
The best prototype of primordial prevention of thyroid disease is adequate iodine nutrition for prevention of iodine deficiency, which is the most preventable cause of mental retardation. Approximately 1.6 billion people worldwide are at risk of iodine deficiency disorders (IDD).
2.1.1.1. Iodine Deficiency Disorders
The term IDD includes all complications of poor iodine nutrition that can be prevented by ensuring the adequacy of iodine intake (5). Iodine deficiency can result in abortions, stillbirths, motor skill disturbances, mental growth defects, impaired cognitive developments, spastic weakness, and paralysis and it affects the child’s learning capacity (lower IQ), deaf-mutism, poor women’s health, and thereby affecting the quality of life of communities and economic productivity (Table 1). The most important effects are related to brain functioning of a child, resulting in decrease in IQ. In addition to the above- mentioned disorders, lack of iodine in the diet may cause decline in the socioeconomic status in communities, whose members have insufficient education and inappropriate productivity. Iodine deficiency in livestock may cause a reduction of milk, meat, and wool production from sheep (7). All these sequelae can be avoided, ensuring a continuous supply of iodine to the entire population through salt iodization, a cost effective, inexpensive, and the most suitable method guaranteeing adequate iodine nutrition (8).
| Period of Life | Disorders |
|---|---|
| Fetus | Abortions, stillbirths, congenital anomalies, increased prenatal mortality, endemic cretinism |
| Neonate | Neonatal hypothyroidism, mental retardation, goiter |
| Childhood | Goitre, retarded physical development, overt and subclinical hypothyroidism, impaired mental function (low intelligence quotient) |
| Adulthood | Goitre, hypothyroidism spontaneous hyperthyroidism in the elderly, impaired mental function |
The Spectrum of Iodine Deficiency Disorders (IDD)
2.1.1.2. Iodine Requirement
Each individual needs only minute amounts of iodine, approximately 150 to 200 microgram daily or a teaspoonful for entire life, to ensure proper synthesis of thyroxin, and regulation of normal growth and development (5).
During pregnancy and lactation, iodine requirement increases and the recommended nutrient intake is set at 250 µg of iodine per day (Box 1). Until recently, it was believed that in regions where ≥ 90% of households had consumed iodized salt for at least 2 years, and the median urinary iodine in school children showed iodine sufficiency, there was no need for iodine supplementation in pregnant and lactating mothers. However, due to low levels of median urinary iodine in these women in some regions with iodine sufficiency, this has been challenged, and the American thyroid association now recommends 150 µg daily iodine supplementation for pregnant and lactating women. In regions without USI or with inadequate iodine supplementation, supplements in the form of daily oral potassium iodide must be provided to meet the requirement of 250 µg iodine daily. In special circumstances, 400 mg of iodized oil could be delivered as a single dose (9).
2.1.1.3. Salt Iodization:
Attempts have been made to improve iodine nutrition in areas of iodine deficiency using iodine supplementation through iodination of bread, water, sugar, oil etc. Although these routes of supplementation have been successful in some regions and communities, it appears that salt iodization is the simplest, cheapest, and most effective means of providing optimum iodine nutrition. Iodized oil preparations may be used when iodized salt is not widely available (10).
2.1.1.4. Global IDD Situation:
In 1991, the world health assembly (WHA) declared iodine deficiency as a major public health problem which should be eliminated by the year 2000. This goal had been endorsed in 1990 by world leaders when they met at the United Nations world summit for children. WHO and UNICEF recommended universal salt iodization (USI) for elimination of IDD in 1992 and 1993. Considerable progress has since been achieved, and it has been estimated that at least 75 million newborns entering the world in 2002 had been protected against the brain damage caused by iodine deficiency. In East Asia and Pacific, 20.8 million (16%) of the global newborns and in South Asia 9 million, (14%) were the highest numbers of newborns who were protected (5, 8).
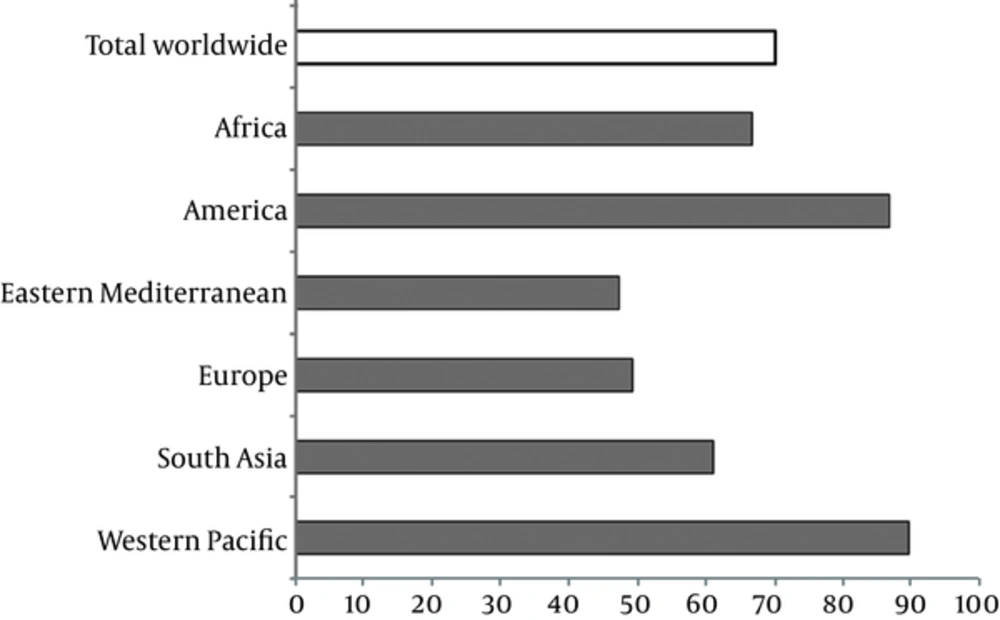
Data made available by WHO shows that 31.5% (264 million) of schoolchildren and 30.6 % (2000 million) of the general population worldwide have insufficient iodine intake (8). The greatest numbers of people with insufficient iodine intake are from Southeast Asia (504 million) and Europe (460 million). Figure 1 displays that household consumption of iodized salt is still less than 50% in the Eastern Mediterranean region and Europe (11).
Proportion of Households with Access to Iodised Salt in Various Parts of the World; Adopted from UNICEF, 2007, Reference (5)
2.1.1.5. Sustainable Elimination of Iodine Deficiency:
Since 1990, there has been a tremendous progress in increasing the amount of adequately iodized salt, and as a result, many countries are now on the threshold of achieving IDD elimination; thus, the emphasis will shift to ensuring that the achievements are well sustained for all time. National (n = 121) or large subnational (n = 31) UIC surveys have been done in 152 countries, representing 98% of the world’s population (12, 13). In 2014, iodine intake was adequate in 112 countries, deficient in 29 countries, and excessive in 11 countries (13). The number of iodine-sufficient countries increased from 67 to 112 during the last 10 years (13). Therefore, the main task in most countries will now be to sustain adequate iodine nutrition for their populations. A limitation of these data is that only a few countries (including IR Iran) have done national UIC surveys in pregnant women, a key target group. Large populous countries that are still iodine deficient include not only developing countries (e.g., Ethiopia, Morocco, and Mozambique) and countries in transition (e.g., Russia and Ukraine), but also several high-income countries (e.g., Denmark, Italy, and the UK) (13). Moreover, in several high-income countries, including the USA and Australia, iodine intakes have decreased in the past 30 years (14). Results of surveys suggest that many pregnant women in both developing and high- income countries, including the UK and the USA, have deficient iodine intakes (15), However, in some countries of the world, iodine supplementation has not been implemented and in many countries, the iodine nutrition supplementation in place is defective and does not reach the recommended levels of daily consumption. Therefore, in 2005 the world health assembly (WHA) once again passed another resolution to encourage the member states to strive for the elimination of iodine deficiency (16).
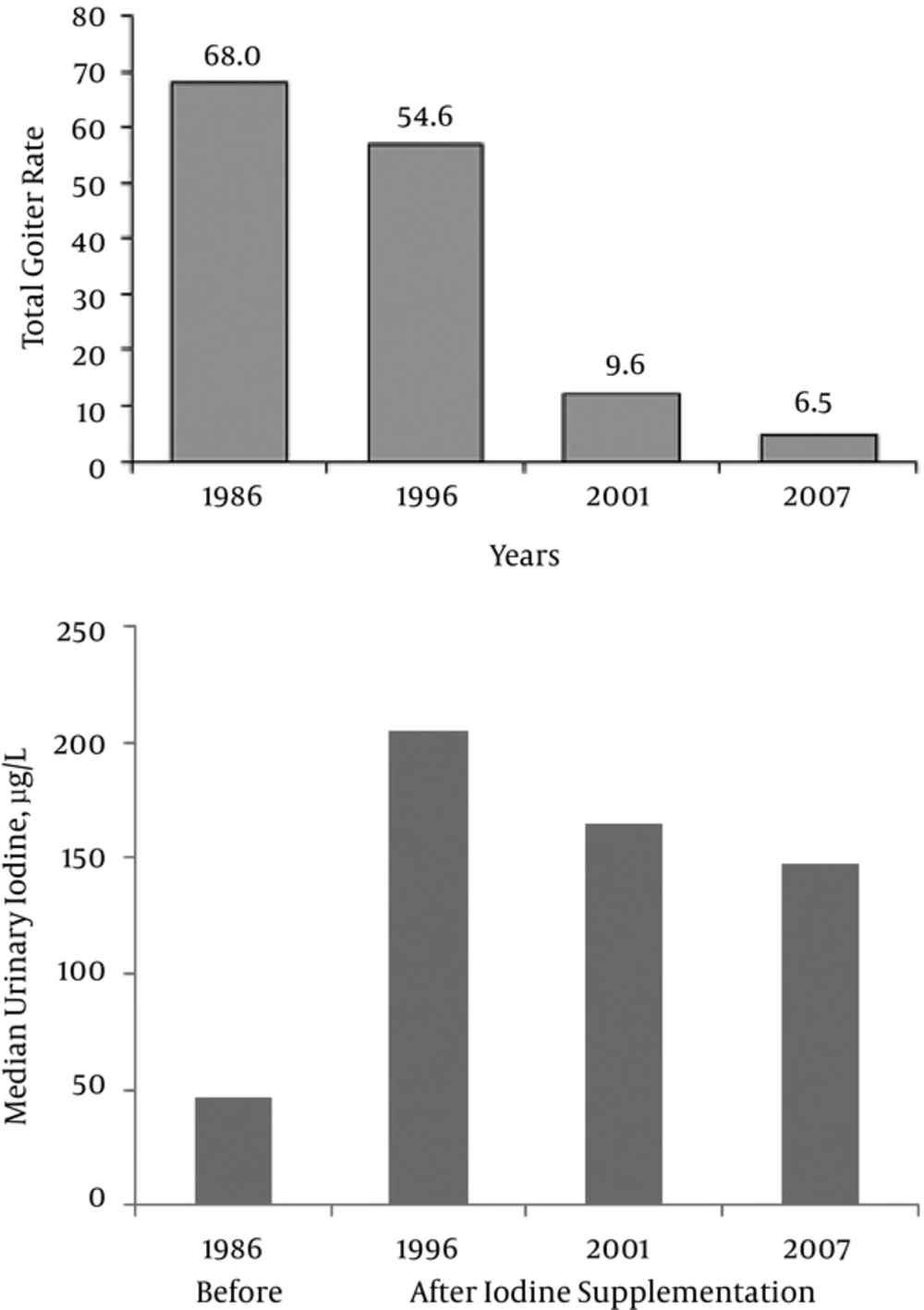
There are many important factors which influence the success and, in particular, the sustainability of an effective IDD elimination program (Box 2). As an example, The Islamic Republic of Iran (IR Iran), a developing country in nutrition transition, has been successful in conducting a sustainable program for more than 2 decades (17, 18). Changes in total goiter rate and median urinary iodine concentration in the past 2 decades in this country are shown in Figure 2.
| Vital Prerequisites for Success of IDD Control Programs |
|---|
| 1. Political commitment |
| 2. Multiple sector involvement in planning and administration |
| 3. Advocacy with the salt manufacturing and trading community |
| 4. Information, education and communication (IEC) campaigns incorporating a social marketing approach |
| 5. Economic and marketing incentives |
| 6. Monitoring of iodine levels in salt |
| 7. Legislation and enforcement |
| 8. Contributions of external donors |
| 9. Program monitoring |
| 10. Leadership |
| 11. National ownership for a sustainable program |
Vital Prerequisites for Success of IDD Control Programs
The lack of success in controlling and monitoring iodine deficiency in many countries presents a dismal picture. Proper estimations of iodine levels in salt factories, retailers, sellers, and households are not regularly done in many countries, and iodine measurement is performed only in a few countries affected by IDD, and others do not have supporting iodine laboratory network. Many countries fail to establish sustainable IDD control programs. The major obstacles faced today are as follow (19):
1- Legislation at government level is ineffective if no enforcement is in place. Many countries of the world lack effective legislation for USI, which has resulted in mild to moderate iodine deficiency and inadequate support for brain development in infants in these countries.
2- Political stability plays a major role in programming successful maintenance of a public health program. Many countries have not been successful in legislating, initiating, or successfully maintaining elimination of IDD, as their public health program has been damaged by political instability, change of officials, and health priorities and programs.
3- Current government policies in many countries favor decreased salt consumption in an attempt to reduce hypertension. This should be considered in programs of iodine supplementation through USI, and while advertising for low salt intake, 40 mg/kg (ppm) or precisely determined appropriate amounts of iodine should be added to the salt, taking into consideration the mean daily salt consumption of the specific populations. The logo should be “take less salt, but take only iodized salt”.
In conclusion, iodine deficiency continues to be a major health problem in many countries. This is mainly due to the inefficient participation of the related policymakers, lack of function of national IDD committees, insufficient collaboration between health care providers, IDD experts, salt producers, communication specialists and consumer associates, and lack of efficient monitoring system for national country programs.
2.1.2. Thyroid Disruptors
Over the past 3 decades, there has been growing awareness that common environmental exposures including a variety of different groups of chemicals may affect thyroid function in humans. A classification of the chemicals that could disrupt thyroid hormone function is shown in Box 3 (20, 21). Neurodevelopment of the fetus and infant are more vulnerable to the permanent effects of thyroid disruptors. Adverse effects related to growth and reproduction may also appear in children and adolescents.
| Classification of Thyroid Disruptors |
|---|
| 1. Inhibition of Iodine Uptake |
| - Perchlorate |
| - Thiocyanates |
| - Nitrate |
| 2. Direct action on thyroid hormone receptor |
| - Polychlorinated biphenyls polychlorinated biphenyls (PCBs) |
| - Bisphenol-A |
| - Triclosan |
| 3. Displacement of T4 from transthyretin |
| - Polybrominateddiphenyl ethers |
| - Hyproxylated PCBs |
| 4. Inhibition of thyroperoxidase activity |
| - Isoflavones |
| 5. Activation of hepatic enzyme (decrease T4 half life) |
| - Orgachlorine pesticides |
| - Dioxins and furans |
| 6. Other substances |
| - Styrenes, perfluorinated compounds, organophosphate pesticides, sunscreens, lead |
Classification of Thyroid Disruptors
Some studies have shown the adverse effects of polychlorinated biphenyl (PCBs) on thyroid function. However, there are only few studies documented on thyroid-disrupting effects of other halogenated compounds, bisphenol A (BPA), UV filters, and phthalates (22-24).
The anions perchlorate, thiocyantae, and nitrate at pharmacologic doses may act as competitive inhibitors of the sodium iodide symporter, but their effects during environmental exposure are not clear (25).
Effects of compounds with direct actions on the thyroid hormone receptors are not yet well understood. Ingestion of high levels of isoflavons may inhibit TPO activity and cause goiter and hypothyroidism, in particular in iodine deficient individuals (26). Quercetin is reported to be the most abundant flavonoid present in fruit and vegetables. It is also available as dietary supplements used as antioxidant, anti-proliferative, and anti-inflammatory agents. Excessive intake of quercetin has the potential of interfering with thyroid function. Quercetin acts as a thyroid disruptor by inhibiting thyroid-cell growth and iodide uptake due to down regulation of sodium/iodide symporter gene expression. A recent study revealed that quercetin may decrease the expression of thyroid peroxidase, thyroglobulin, and the thyrotropine receptor genes. Radioiodine uptake was significantly decreased after 14 days of quercetin treatment in rats, indicating the inhibitory effect of this substance on thyroid function (27).
Cyanide is one of the major environmental pollutants termed “thyroid disruptors”. In one study, cyanide induced hyperthyroidism in male Wistar rats (28). Resveratrol, a polyphenol found in grapes and berries, which has antioxidant, antiproliferative and anti-inflammatory properties, causes inhibition of sodium/iodide symporter gene expression and function in the rat thyroid and may act as a thyroid disruptor, but its use as a supplement needs further evaluation (29).
Alterations of thyroid hormone secretion after long-term exposure to low doses of DDT through disruption of thyroxine secretion by thyrocytes have been reported. The DDT treatment in a low dose may induce similar changes observed during the development of hypothyroidism in conditions of iodine deficiency (30, 31).
In general, effects of the most potential thyroidal disruptors on thyroid function at environmental exposure levels remain unclear. Multiple exposures of disruptors could have additive or synergistic effects, data which should be confirmed by future studies. Unavoidable lifelong human exposure to mixtures of such environmental chemicals raises serious concerns about their potential adverse effects on thyroid function. Two recent articles have described some specific effects of disruptors on thyroid function of pregnant women and neurodevelopment of offsprings. In one study, higher end maternal urinary levels of perchlorate have been associated with the lowest IQs of offsprings (20). In another study, perfluroalkyl acids exposure has been accompanied with high serum TSH and low FT4 in TPOAb positive pregnant women (22). In the above-mentioned situations, where the evidence is not complete and there might be a suggestion of harm, the precautionary prevention may be used to avoid public health risks from processes or products (32).
2.1.3. Risk Factors of Thyroid Autoimmunity
Antibodies to thyroglobulin (TG) and thyroperoxidase (TPO) are present in both Hashimoto’s thyroiditis and diffuse toxic goiter up to 7 years before the diagnosis (33). Lymphocyte infiltrates in the thyroid at autopsy have been reported in people with low levels of antibodies but without clinical disease (34). Many reasons have been proposed for the development of autoimmunity including thyroid autoimmunity, and understanding these factors may be useful in preventing occurrence of thyroid autoimmunity.
2.1.3.1. Immunity Factors
Immunity factors may include abnormal presentation of antigens caused by cell destruction, invasion of virus, abnormal antigen (degraded or denatured normal antigen) produced by a malignancy, or cell damage by viral attack, cross-reacting bacterial, or viral epitopes, e.g., Yersinia enter colitica (35, 36). Other etiologic or pathophysiologic process are as follow: Somatic mutation of a TCR rarely leading to a clone of self-reactive cells, inheritance of specific HLA, TCR, or other genes that code for antigen presentation proteins, aberrant T cell or B cell, failure of clonal deletion leaving self-reactive T cells, failure of normal maturation of immune system, allowing autoreactivity of fetal T and B cells, and polyclonal activation of T or B cells, leading to B cells producing self-reactive Ig, and somatic mutation of B cell Ig genes (36).
Regarding thyroid stimulating immunoglobulin (TSI), which is clonally restricted to the V gene, it has been suggested that Graves’ disease may be due to the inheritance of an etiologically critical V gene encoding TSI. Also, as a primary event, epithelial cells (TECs) could express MHC class II molecules, and thereafter may function as APCs including antigens on their cell surface (36).
The coexistence of autoimmune thyroid disease (AITD) and other autoimmune diseases has suggested intrinsic abnormality in immune regulation. AITDs have associations with pernicious anemia. Circulating antigastric antibodies are present in 45% of the patients with autoimmune thyroiditis (37), and the reverse association is almost as strong (38). Approximately 14% of patients with pernicious anemia have hypothyroidism. The prevalence of pernicious anemia is also increased in patients with myxedema (39). Celiac disease is 3 times more common in patients with AITD; the autoantibodies against transglutaminase bind to thyroid cells and may play a role in thyroid disease pathogenesis (40). An associations of Sjogren’s syndrome, systemic lupus erythematous (SLE), and rheumatoid arthritis with AITD have been reported (41, 42). There is also a high frequency of antibodies to nucleus, smooth muscle, and single-stranded DNA (26% - 36%) in AITD (43). A meta-analysis has reported an odds ratio of 1.7 for AITD in patients with multiple sclerosis (44). Type 1 diabetes mellitus, AITD, and Addison’s disease occasionally coexist as the autoimmune polyglandular syndrome (APS) Type 2 (45), an autosomal dominant disorder, which may be associated with vitiligo, celiac disease, premature ovarian failure, myasthenia gravis, and chronic active hepatitis (46, 47). Autoimmune thyroid disease can be an infrequent feature of the much rarer APS Type 1 (48). There is no association between mutations in the AIRE (auto immune regulation) gene, which causes APS Type 1 and sporadic AITD (49).
2.1.3.2. Environmental Factors Inducing Thyroid Autoimmunity
Incomplete concordance seen in monozygotic twins or other siblings of individuals with AITD points to the strong evidence for an important role of environmental factors. The rise in childhood Graves’ disease in Hong Kong, the steady rise in AITD in Calabria, Italy, and Austria, and the changes in the rates of histologically diagnosed Hashimoto’s thyroiditis over a 124-year period (50-53), point to the environmental influences in AITD incidence (50-53). Some studies have revealed a higher prevalence of thyroid autoimmunity in children raised in higher socioeconomic environments (54); the data are in line with the hygiene hypothesis that early exposure to infections may deviate the immune system from Th2 responses like allergy and autoimmunity.
Administration of IL-2 for the treatment of cancer (55), INF-α, and other cytokines as well as antiretroviral therapy for HIV (56) may lead to the production of antithyroid antibodies and decreased thyroid function, which may be associated with a better tumor response. Interferon-β 1b treatment has no significant adverse effect on AITD (57), however, long-term follow- up studies show that one fourth of patients with multiple sclerosis treated with this cytokine may develop AITD within the first year of treatment (58). Recurrence of thyrotoxicosis following attacks of allergic rhinitis (59) may be due to a rise in endogenous cytokines, and the association of raised IgE levels with newly diagnosed Graves’ disease may be mediated by preferential Th2 activation (60).
Cigarette smoking is associated with Graves’ disease and thyroid disease (61)although smoking is also reported to be associated with decreased incidence of hypothyroidism (62). Environmental tobacco smoke induces allergic sensitization in mice, which is associated with rise in production of Th2 cytokines but a decrease in Th1 cytokines by the respiratory tract (63). Modulation of cytokines may contribute to the worsening of ophthalmopathy with smoking and the opposite effect prevails in hypothyroidism and smoking exposure; a lower prevalence of thyroid autoantibodies in smokers was documented (64). Smoking cessation may induce a transient rise in AITD (65). Anatabine, an alkaloid found in tobacco, ameliorates experimental auto immune thyroiditis and reduces thyroglobulin (TG) antibody levels in humans with Hashimoto’s thyroiditis (66).
More general environmental pollutants such as polychlorinated biphenyls (67) and pesticide use, in particular the fungicides Benomyl and Maneb/Mancozeb, have been associated with increased odds of developing thyroid dysfunction. Brazilians have shown that thyroid autoantibodies and Hashimoto’s thyroiditis are more frequent in individuals living close to a petrochemical complex than in controls (68).
The role of dietary iodine has been established in animal models of AITD and evidence exists for a similar role in humans (69-72). It has been shown that although iodide may exacerbate thyroiditis in NOD mice, it did not affect the production of TSH-R antibodies, indicating that change in Graves’ disease may not be related to TSH-R. Iodine affects several aspects of the autoimmune response and also stimulates production of the chemokine CCL2, CXVL8, and CXCL14 in follicular cells (73). Therefore, iodine may induce AITD through upregulation of chemokines, which attract lymphocytes into the thyroid.
High dose selenium intake decreases TPOAb levels in women with AITD (74), as shown by the significant inverse association between 25 (OH) D concentrations and TPOAb levels in Indians (75). AITD is also increased in same-sex marriage (76). The pathophysiology behind above findings is not clear.
The importance of stress in etiology of Graves’ disease has been suggested (77). However, stress is not associated with TPOAb development in women (78). The pathway whereby stress may alter thyroid autoimmunity is unclear (79, 80). It has also been reported that moderate consumption of alcohol may have a protective effect with regards to AITD (81-83).
In summary, diversity of environmental factors and their possible effects on various genetic backgrounds have made it difficult to establish the relative importance of each factor in AITD. Viral and other infections are also considered as environmental factors (36).
2.2. Primary Prevention of Thyroid Disease
For many years, thyroidologists have been researching factors other than iodine that may affect thyroid health; of various factors that have been documented as having some effects on thyroid size and function, tobacco abuse and alcohol are thought to be more prominent.
2.2.1. Tobacco Smoking
Smoking is one of the leading preventable causes of various health derangements and death (84). Cigarette smoking introduces a large number of chemical substances to the human body that may affect the thyroid gland in different ways. The competitive inhibitory effect of thiocyanate on the sodium-iodine symporter (NIS) worsens iodine deficiency (85). Tobacco smoking causes a small decrease in serum TSH and an increase in serum free T4 (86). The effects of smoking on increased risk of Graves’ hyperthyroidism, Graves’ orbitopathy, in particular, are well- recognized (87).
There are conflicting results regarding the association between smoking and hypothyroidism. It has been shown that incidence of hypothyroidism increases following cessation of cigarettes smoking; in addition, the prevalence of circulating TPOAb is lower in smokers as compared to nonsmokers (88), which is coupled with the appearance of thyroid antibodies in serum following smoking cessation (89) and accompanied by a transient rise in the incidence of overt autoimmune hypothyroidism (65). The exact pathophysiology of these changes has not been elucidated. Other studies have suggested that smokers may have a lower risk of thyroid cancer than nonsmokers (90).
It can be concluded that smoking cessation, which is recommended for community health, may lead to a decline in the overall prevalence of thyroid disease; however, populations may experience a shift in the type of thyroid disease (6).
2.2.2. Alcohol Consumption
Alcohol abuse leads to considerable morbidity and mortality. However, scientists from Denmark report that a reduction in the risk of Graves’ disease with hyperthyroidism may be seen with moderate alcohol consumption (81), whereas others report conflicting results (91, 92); 3 studies found no effect of alcohol consumption in Graves’ patients, while 1 showed a decrease risk in women but not in men.
2.2.3. Thyroid Autoimmunity (Positive Thyroid Antibodies)
It has been recommended that the presence of thyroid antibodies per se is not a reason to initiate thyroid hormone therapy. Despite the fact that the presence of antibodies alone can cause thyroid-related symptoms, few endocrinologists treat those with positive antibodies and TSH within the normal range who feel unwell. The presence of thyroid antibodies alone can affect fertility or the ability to maintain a pregnancy (93); therefore, some thyroidologists treat euthyroid pregnant women with positive TPOAb. Thyroid autoimmunity is characterized by infiltration of sensitized T lymphocytes in the thyroid gland and increased thyroid autoantibodies in the serum; it is an inherited defect in immune surveillance, which leads to alteration of presenting antigens in the gland, or abnormal regulation of immune responsiveness (94, 95). Clinical diagnosis of AITDs is usually made by the elevated levels of TSH-receptor antibodies (TSHRAb), antimicrosomal thyroid peroxidase antibodies (TPOAb), or anti-thyroglobulin antibodies (TgAb), with 90% of patients with AITD having elevated antibody titers, which generally persist, although many of these patients are clinically and biochemically euthyroid.
Rates of positive TgAb and TPOAb in populations vary from 8% to 16% in various surveys (96, 97). In patients with subclinical hypothyroidism, TPOAb positivity helps predict progression to overt hypothyroidism. Approximately 4.3% and 2.6% of those with and without elevated TPOAb develop hypothyroidism yearly, respectively (98). Therefore, some professional societies and clinical endocrinologists endorse measurement of TPOAb in patients with subclinical hypothyroidism; assessment of TPOAb in patients with diffuse and firm goiter could identify autoimmune thyroiditis and predict any possibility of future hypothyroidism (99).
Patients with non-immunologic multinodular goiter rarely progress to hypothyroidism; however, it is important to separate patients who carry nodular variant of AITD because these patients may have be at a significant risk of hypothyroidism; in addition, they may need to have fine-needle aspiration biopsy to exclude malignancy (52).
Measurement of TSHRAb, which may act as a TSH agonist or antagonist, may be indicated under several conditions. The main indication of measurement of thyroid stimulating immunoglobulin (TSH) and/or TSH binding inhibitory immunoglobulin (TBI) is in pregnant women with a history of Graves’ disease (irrespective of present thyroid function), as these tests predict fetal and neonatal hyperthyroidism (100); the time of measurement differed in various situations; in addition to the 28th weeks of pregnancy, earlier measurement of TSHRAb may also be indicated (101).
3. Closing Remarks and Direction of Future Research
A wide range of public health and medical considerations encounter the prevention of thyroid disease, a subject which has received far too little attention worldwide from policymakers and medical professionals alike.
The main issue for primordial prevention of thyroid disease is proper iodine nutrition. Iodine deficiency is a major public health problem that is still prevalent in many countries of the world, in particular, in some European countries. Even in the so called “iodine sufficient” countries, iodine nutrition may be inadequate for pregnant women. Of factors affecting primary prevention, cigarette smoking has definite but variable effects on the thyroid economy. Successful campaigns against smoking may decrease benign thyroid disease and Graves’ orbitopathy although this may be countered by increase in TPOAb, transient hypothyroidism, and a slight increase in the cases of thyroid cancer. Future researches are needed to find the best approach to prevent the presence of thyroid antibodies.