1. Context
The metabolic syndrome (MetS) is the concurrence of multiple metabolic abnormalities including centrally distributed obesity, decreased high-density lipoprotein cholesterol (HDL-C), elevated triglycerides, elevated blood pressure (BP), and glucose intolerance (1). It is frequently associated with cardiovascular disease (CDV), high all-cause mortality (2), and represents an enormous global public health problem. It is believed that chronic inflammation in MetS is an important common factor underlying the state of insulin resistance, atherosclerosis and CVD (3).
Patients with end-stage renal disease (ESRD), especially those on dialysis, have higher risk of mortality due to cardiovascular disease than the general population (4). In contrast, studies reveal that patients with renal insufficiency or dialysis patients who had higher body mass index (BMI) might have a lower risk of mortality compared to those who had low BMI (5, 6). This inverse relationship between obesity and mortality in the ESRD population is called the “obesity paradox”. A literature review on this topic provides summaries of studies evaluating the association between BMI and mortality in hemodialysis (HD) and peritoneal dialysis (PD) patients. Most studies concluded that high BMI, adiposity, and fat mass were associated with increased survival in ESRD patients. Obesity, particularly the excess accumulation of visceral fat is an important component of MetS, which has been associated with increased mortality in the general population. Therefore, it is of considerable clinical interest to understand to which extent the obesity factor interferes with the increased survival rates in ESRD patients. It is still unknown whether ESRD population with Mets has increase or decrease mortality and cardiovascular disease.
This systematic review and meta-analysis of longitudinal studies was performed to compare overall mortality, CVD death and cardiovascular disease events (CVE) in ESRD patients with and without the diagnosis of MetS. We hypothesize that metabolic effects and chronic inflammatory state in MetS may have a negative impact in ESRD patients.
2. Evidence Acquisition
This systematic review and meta-analysis was conducted and reported according to the Meta-analysis of observational studies in epidemiology statement (7) and was registered in PROSPERO (registration number: CRD42015023268).
2.1. Search Strategy
Both authors (AS, SU) independently searched published studies indexed in the MEDLINE, EMBASE, and the cochrane central register of controlled trials (CENTRAL) in The cochrane library from date of inception to March 2017. References of all selected studies were also examined. The following main search terms were used: metabolic syndrome, insulin resistance, obesity, renal insufficiency, end stage renal disease and dialysis. The full search terms used are detailed in the supplementary file Appendix 1.
2.2. Inclusion and Exclusion Criteria
This review included all published randomized controlled trials and prospective or retrospective cohort studies that assessed the association of metabolic syndrome with mortality or cardiovascular disease outcomes (death, new CVE) in patients with ESRD. Reviews, case reports, letters, commentaries, abstracts, and unpublished studies were excluded. These were excluded because the quality of studies could not be evaluated properly.
We included studies that used the following definitions of metabolic syndrome: NCEP-ATP III criteria (8, 9), modified NCEP-ATP III criteria (10), IDF definition (11), or WHO criteria (12). Information for each criterion is available in Additional Table 1.
| Study | Design | Follow-Up (Months) | Country | Participants (n) | MS Criteria | HD (Months) | Factors Adjustment | |
|---|---|---|---|---|---|---|---|---|
| MS | Non-MS | |||||||
| Yang 2007 | Prospective cohort | 36 | Taiwan | 108 | 127 | Modified Asian criteria of NCEP ATP III | 50.8 | |
| Xie 2012 | Prospective cohort | 36-42 | China | 46 | 111 | IDF | 44 | |
| Wu 2011 | Prospective cohort | 36 | Taiwan | 46 | 45 | NCEP, IDF | 25 | Age, hemoglobin, albumin, hs-CRP |
| Vogt 2014 | Prospective cohort | 64 | Brazil | 50 | 49 | NCEP ATP III, IDF | 42 | Age, sex, serum creatinine |
| Prasad 2013 | Prospective cohort | 24 | India | 84 | 79 | Modified Asian criteria of NCEP ATP III | PD at least 3 months | Age, gender, comorbidities, serum albumin, peritonitis |
| Park 2010 | Prospective cohort | 60 | Korea | 50 | 56 | NCEP ATP III | CAPD 83.4 | Age, sex, albumin, hematocrit and dialysis duration |
| Liao 2011 | Prospective cohort | 49.2 | Taiwan | 90 | 132 | NCEP ATP III | PD at least 3 months | History of smoking, pre-existing CVD, residual GFR, LDL-C, serum albumin, CRP |
| Johnson 2007 | prospective, open-label randomized controlled trial | 24 | Australia | 61 | 139 | WHO | HD 1.5 years PD 0.9 year | Residual renal function, adiponectin, albumin, calcium, phosphate, free fatty acid, homocysteine, CRP |
Abbreviation: CVD, cardiovascular disease; CRP: C-reactive protein; Non-MS, non-metabolic syndrome; HD, hemodialysis; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; LDL-C, LDL cholesterol; MS, metabolic syndrome; PD, peritoneal dialysis.
We included participants aged 18 years or older who had ESRD (defined as eGFR or creatinine clearance less than 15 mL/min/1.73 m2, or receiving HD or PD). Participants who received renal transplantation were excluded from the analysis. The primary outcome was overall mortality and secondary outcomes were death due to CVD and CVE. CVE included ischemic heart disease (myocardial ischemia or unstable angina or myocardial infarction documented by clinical diagnosis in medical record or angiography), congestive heart failure, sustained arrhythmias (documented by electrocardiography), cerebrovascular events (documented by brain imaging study), peripheral vascular disease (documented by angiographic or sonographic detection of ≥ 50% stenosis for the major peripheral arteries of the lower extremities), decompensated heart failure/cardiogenic shock, and sudden cardiac death.
2.3. Data Extraction
Both authors independently reviewed titles and abstracts of all citations that were identified. After all abstracts were reviewed, data comparisons between investigators were conducted to ensure completeness and reliability. The inclusion criteria were independently applied to all identified studies. Differing decisions were resolved by consensus.
Full-text versions of potentially relevant papers identified in the initial screening were retrieved. If multiple articles from the same study were found, only the article with the longest follow-up period was included. Data concerning study design, participant characteristics, metabolic syndrome defining criteria, and outcome measures were independently extracted. We contacted the authors of the primary reports to request any unpublished data. If the authors did not reply, we used the available data for our analyses.
2.4. Assessment of Bias Risk
A subjective assessment of methodological quality for randomized-controlled trial was conducted by both authors on the following items, in which each component was categorized as having high, low or unclear risk of bias: selection bias (random sequence generation, allocation concealment), blinding (participants and personnel, outcome assessment), and reporting bias (incomplete outcome data, selective reporting). The quality of observational cohort study was evaluated by the same authors using the newcastle-ottawa scale (NOS) (13). In brief, the NOS is a quality assessment tool for non-randomized study. It uses a “star system” based on three major perspectives: the selection of the study groups (0 - 4 stars), the comparability of the groups by controlling for first and second most relevant factors (0 - 2 stars), and the ascertainment of outcome of interest (0 - 3 stars). A total score of 3 or less was considered poor, 4 - 6 was considered moderate, and 7 - 9 was deemed high quality. We empirically excluded studies from our study if they had poor quality. Discrepant opinions between authors were resolved by consensus.
2.5. Statistical Analysis
We performed meta-analysis of the included studies using comprehensive meta-analysis 3.3 software from Biostat, Inc. We used reporting number of event or hazard ratio (HR) from univariate or multivariate analysis in each study. We then calculated pooled estimate risk ratio (RR) with 95% confidence intervals (CI) to report the estimate for the outcomes of all-cause mortality, CVD death and CVE. We excluded studies from meta-analysis and only presented the result with narrative description when there were not sufficient comparable data available for outcome of interest. The heterogeneity of effect size estimates across these studies was quantified using the Q statistic, its p-value, and I2. The Q statistic compared the observed between-study dispersion and expected dispersion of the effect size, and was expressed in P value for statistical significance. An I2 is the ratio of true heterogeneity to total observed variation. An I2 of 0% to 40% was considered to exclude heterogeneity, of 30% to 60% was considered to represent moderate heterogeneity, of 50% to 90% was considered to represent substantial heterogeneity, and of 75% to 100% was considered to represent considerable heterogeneity (14). Subgroup analysis and meta-regression was performed to find the source of heterogeneity. Publication bias was assessed using funnel plot, Egger’s regression test and its implications with the fail-safe n and the trim and fill method (15) (P < 0.10 was considered significant).
3. Results
3.1. Description of Included Studies
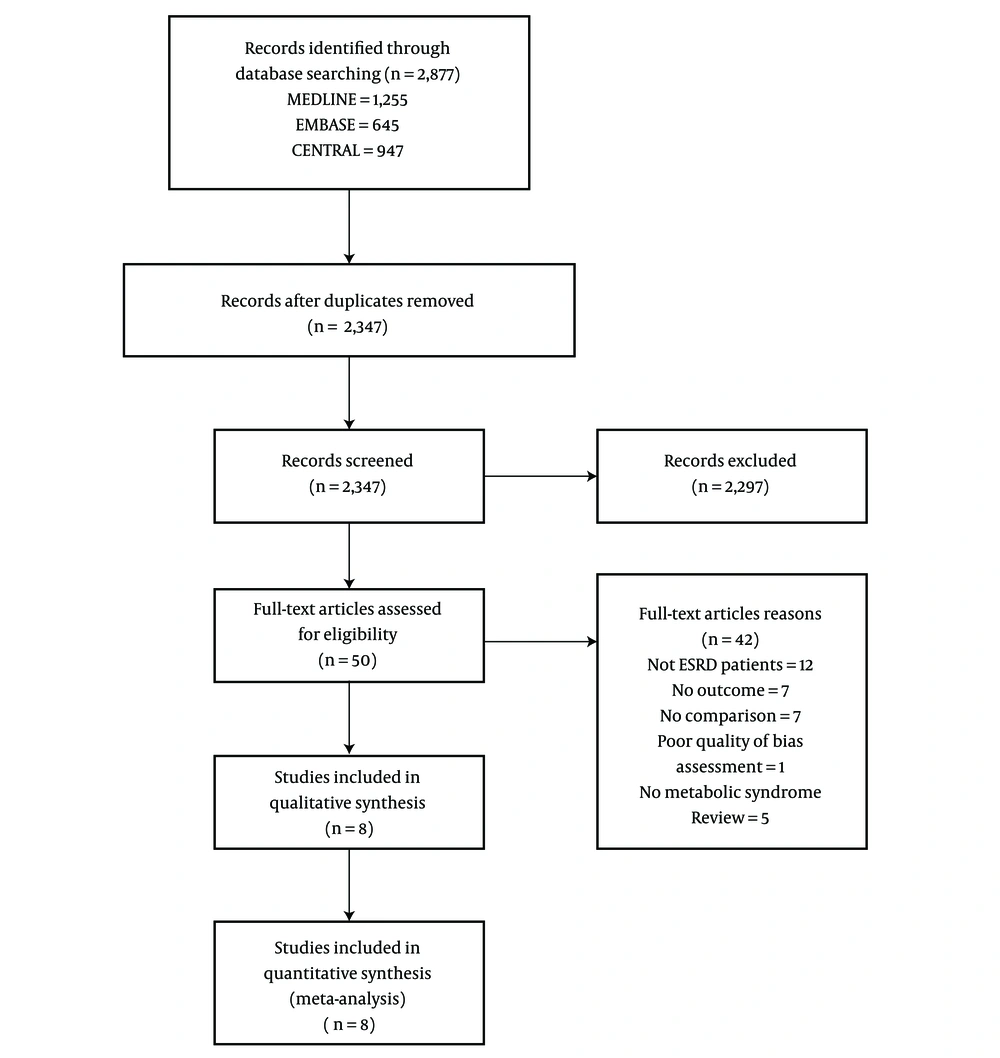
The initial search yielded 2,347 articles (Figure 1); 2,297 were excluded because they were not RCTs or cohort studies (1,011 articles), did not involve ESRD participants (535 articles), or did not have outcomes of interest (751 articles). A total of 50 articles underwent full-length review. Data were extracted from eight involving total of 790 ESRD participants for qualitative and quantitative analysis. The included studies varied in sample size (91 to 235), criteria for diagnosing metabolic syndrome, type of dialysis (HD and PD), and duration of dialysis (at least 24 months to 60 months). Among the eight included studies, seven were prospective cohort studies, and one was an RCT. The characteristics of the eight extracted studies included in this review are outlined in Table 1.
Of these, seven studies were included in the meta-analysis for all-cause mortality (16-21), four studies in the analysis for CVD death (20-23), and two studies in the analysis for CVE (19, 23). All studies reported estimates as hazard ratio or number of events.
3.2. Risk of Bias of Included Studies
The quality of all included studies is presented in Table 2. For cohort studies, seven studies had a total score of 7 - 9. Risk of bias for RCT study (Johnson et al.) was determined for selection bias (low risk), blinding (low risk), and reporting bias (low risk).
| Study, Year | Selection | Comparability | Outcome | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the sample | Non-exposed group from same community | Outcome of interest was not present at start of study | Validated measurement tool | Comparability of different samples on the basis of the design or analysis | Assessment of outcome | Adequate follow up | Completeness of follow up | ||||||
| Truly representative | Somewhat representative | Study controls for important factor | Study controls for any additional factor | Independent blind assessment | Record linkage | Complete | Small number loss follow up | ||||||
| Yang 2007 | * | * | * | * | * | * | * | 7 | |||||
| Xie 2012 | * | * | * | * | * | * | * | 7 | |||||
| Wu 2011 | * | * | * | * | * | * | * | * | * | 9 | |||
| Vogt 2014 | * | * | * | * | * | * | * | * | * | 9 | |||
| Prasad 2013 | * | * | * | * | * | * | * | * | * | 9 | |||
| Park 2010 | * | * | * | * | * | * | * | * | 8 | ||||
| Liao 2011 | * | * | * | * | * | * | * | * | * | 9 | |||
| Johnson 2007 | RCT (Low risk of selection, blinding, and reporting bias) | ||||||||||||
3.3. Meta-Analysis Results
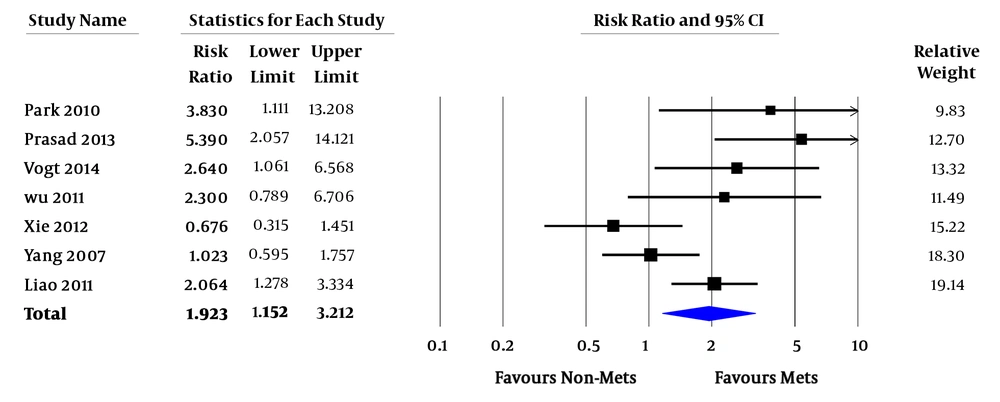
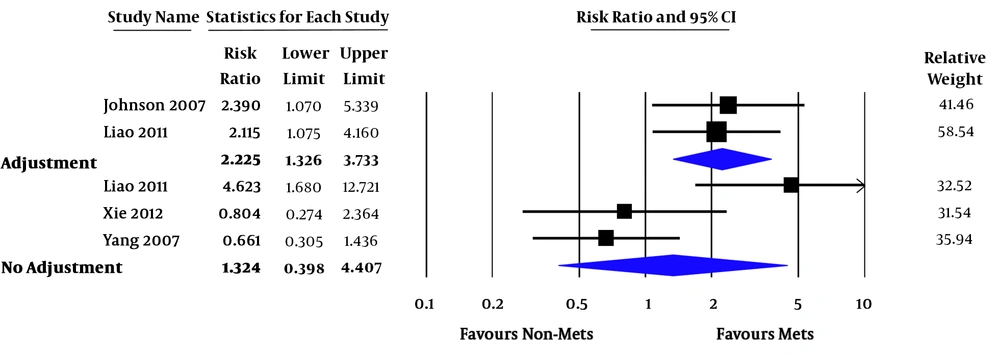
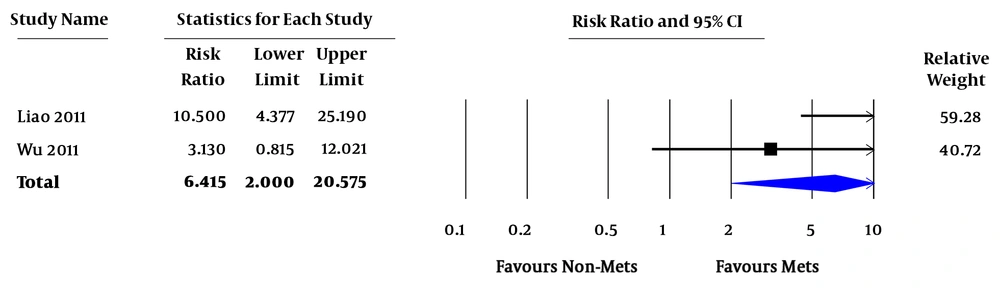
Relative risk (RR) of all-cause mortality varied from 0.7 - 7.4. In the pooled analysis, MetS was associated with increased risk of all-cause mortality in ESRD patients (pooled RR = 1.92; 95% confidence interval [CI] 1.15 - 3.21; P = 0.01; I2 = 66%) (Figure 2). RR of CVD death varied from 0.3 - 12.7. In the pooled analysis of studies with factors adjustment, MetS was significantly associated with CVD death (pooled RR = 2.23; 95% CI 1.33 - 3.73; P = 0.002; I2 = 0%). In the pooled analysis of studies without factors adjustment, MetS was not significantly associated with CVD death (pooled RR = 1.32; 95% CI 0.40 - 4.41; P = 0.65; I2 = 79%) (Figure 3). RR of CVE ranged from 2.4 - 3.1. In the pooled analysis, MetS was significantly associated with cardiovascular event (pooled RR = 6.42; 95% CI 2.00 - 20.58; P = 0.002 (Figure 4).
3.4. Exploration of Heterogeneity
There was significant statistical heterogeneity between the included studies in the pooled analysis of all-cause mortality (I2 = 71%, P = 0.004) and CVD death (I2 = 72%, P = 0.014). We conducted subgroup analyses and univariate meta-regression for all-cause mortality. Subgroup analysis of studies that used NCEP or modified NCEP criteria (RR = 2.45; 95% CI 1.03 - 5.83) versus studies that used other criteria (RR = 1.58; 95% CI 0.81 - 3.09) found a non-significant difference in risk of mortality (Pbetween = 0.56). In the subgroup of type of dialysis, there is difference in mortality (Pbetween = 0.01) between studies of patients with HD (RR = 1.32; 95% CI 0.72 - 2.39), and studies of patients with PD (RR = 4.74; 95% CI 2.22 - 10.43). In a subgroup analysis level of adjustment (supplementary file Appendix 2), there is no difference in mortality (Pbetween = 0.21) between studies with factors adjustment (RR = 3.04; 95% CI 1.61 - 5.75), and studies without factors adjustment (RR = 2.14; 95% CI 1.05 - 4.37). In univariate meta-regression, age (beta = -0.12, P = 0.0003), type of dialysis (beta = 1.07, P = 0.02), and HDL-C (beta = 0.05, P = 0.003), are the significant predictors of risk of mortality. Duration of follow up (P = 0.94) and duration of dialysis (beta = 0.01, P = 0.60) were not predictors of risk of mortality.
3.5. Sensitivity Analysis
To assess the stability of the results of the meta-analysis of all-cause mortality, sensitivity analysis was conducted by excluding one study at a time. As a result, the pooled RR was not significantly altered, indicating thus that our results were robust.
3.6. Publication Bias
The funnel plot suggested the possible presence of publication bias studies with significant results toward right of pooled RR. The Egger’s test was not significant (P = 0.33). The fail-safe n for this pooled analysis was 26 (the number of studies reporting null effect that are needed to make the results insignificant). Using the trim and fill methods in random-effects model, the imputed RR for all-cause mortality was 1.49 (95% CI 0.88 - 2.51).
4. Discussion
Our systematic review and meta-analysis demonstrates that in the ESRD population, patients with metabolic syndrome (MetS) have higher risk of all-cause mortality compared with patients without metabolic syndrome. This risk is more prominent in the PD than in HD population, and age, and HDL-C are significant predictors of mortality. We also found that patients with MetS have a significantly higher risk of cardiovascular disease events than those without MetS, and higher death due to cardiovascular disease in studies with factors adjustment. However, there was no difference in the relative risk of death due to cardiovascular disease between groups in the studies without factors adjustment.
In the past two decades, multiple large prospective studies of patients with ESRD requiring either HD or PD found a significantly higher survival rate in higher BMI (overweight, obesity class I or II) compared to those who had normal BMI or underweight (24, 25). It is more prominent and persistent in HD than PD (26). Potential underlying mechanisms of this “obesity paradox” include benefits of lean body mass and body fat mass in higher BMI (27) causing less protein-energy wasting in dialysis patients, less complications from hypotension, renin-angiotensin system stimulation (28), and risk of fluid retention during HD (29). Another hypothesis that supports this association is time discrepancy between mortality risk from dialysis and from CVD risk factors in obesity. Since most ESRD patients on dialysis die within 5 years of starting dialysis treatment (30), it may be difficult to see long-term effects of obesity on CVD and mortality. Results from these studies have led to a recommendation for dialysis patients to maintain higher BMI.
Most studies used BMI as a predictor of mortality in ESRD patients. However, there is evidence that other anthropometric measurements such as waist circumference, waist-hip ratio or metabolic risk factors such as ratio of triglyceride and HDL, visceral adipose tissue area, and metabolic syndrome criteria are better predictors of CVD risk and mortality than BMI (31, 32). Since high BMI could suggest high muscle mass, body fat mass or visceral fat mass, most studies of longitudinal CVD risk factors usually use aforementioned measurement as predictors of outcome.
This meta-analysis provided evidence that contrasts with existing findings. We found that metabolic syndrome, independent of source of the diagnostic criteria, increases mortality and CVEs in ESRD patients. The most significant components of MetS that predict mortality are TG and HDL, consistent with studies in the general population to predict individuals with high risk of CVD. This result underlies the importance of metabolic risk factors cluster and insulin resistance in ESRD population. Evidence in the general population suggests that lifestyle modification and surgical intervention to decrease metabolic risk factors improved survival rate and risk of CVD (33). Therefore, we believe dialysis patients with MetS would benefit from CVD risk factors reduction.
It is important to note that subgroup analysis of type of dialysis suggests that risk of mortality in PD patients is higher than HD patients. Studies found that survival advantage associated with large body size seemed to be less likely in PD than HD patients (26). We speculate that this might be because in PD patients (34), 1.5% - 4.25% of dextrose is included in their peritoneal dialysate and one-half of it is absorbable. While in HD, only 1% of dextrose is added in the dialysate. Therefore, it is conceivable that benefits in mortality reduction of high body mass or fat mass in PD may be attenuated by this difference in calories from dextrose.
There are several limitations in our meta-analysis, and thus our results should be interpreted with caution. First, this is a meta-analysis of observational studies. Although they are prospective cohort studies, whether there is a reduction in metabolic risk factors in MetS and whether these changes will lead to a decreased risk in mortality could not be answered. Secondly, we included a relatively small number of studies in the meta-analysis. Although there are several studies addressing the metabolic syndrome in ESRD that were included in the qualitative analysis, many could not be included in meta-analysis because they were either of low quality or had outcomes that could not be computed.
Thirdly, there is high heterogeneity between studies in the meta-analysis. Potential sources of heterogeneity assessed by subgroup analysis and meta-regression were type of dialysis, age, HDL-C, and triglyceride levels. In addition, there are publication biases toward those studies of higher risk in the MetS group. This is certainly made worse because we excluded unpublished studies, case reports, letters, and communications.
4.1. Conclusion
Metabolic syndrome is a risk factor for mortality in ESRD patients, especially those who are on PD. Our findings also suggest that the presence of metabolic syndrome in ESRD patients does not confer higher survival. Both physicians and patients should be aware of this metabolic risk cluster in patients and pay particular attention to those who have abdominal obesity. Perhaps, an evaluation using imaging techniques should be recommended in some cases, as it might help to elucidate whether the excess fat accumulation is in the visceral or the subcutaneous area, or in both. Future research should be done to examine whether improvement in each individual metabolic syndrome component will change mortality or CVD outcomes in ESRD patients.