1. Introduction
Ganglioneuroblastoma is an uncommon malignant tumor of the sympathetic nervous system. It is considered a disease of childhood and rarely occurs in adults (1). Fewer than 50 cases of ganglioneuroblastoma in adults have been reported in the literature and only 18 cases have been observed in the adrenal gland. We report of another patient with an adrenal ganglioneuroblastoma.
2. Case Presentation
A 38 -year-old man, with a 3-year history of hypertension and diabetes, was evaluated for abdominal discomfort and urinary tract infection symptoms. He experienced frequency, dysuria, and mild hematuria. He had no history of urologic or chronic medical disorders. Physical examination showed no tenderness and pain elicited by percussion in the kidney areas. The patient underwent a sonography, which indicated absence of kidney stone.
A right adrenal mass was discovered on abdominal sonography. The patient was referred to the endocrinology department for investigation of an adrenal incidentaloma. On admission, physical examination revealed a healthy appearing male, with a blood pressure of 158/88 mmHg, a pulse of 88 beats per minute and his body mass index (BMI) was 25.5. There were no palpable abdominal masses. Other routine examinations were normal. His drug history included metformin 1500 mg daily, atorvastatin 20 mg daily, losartan 50 mg twice daily, and hydrochlorothiazide 25 mg daily.
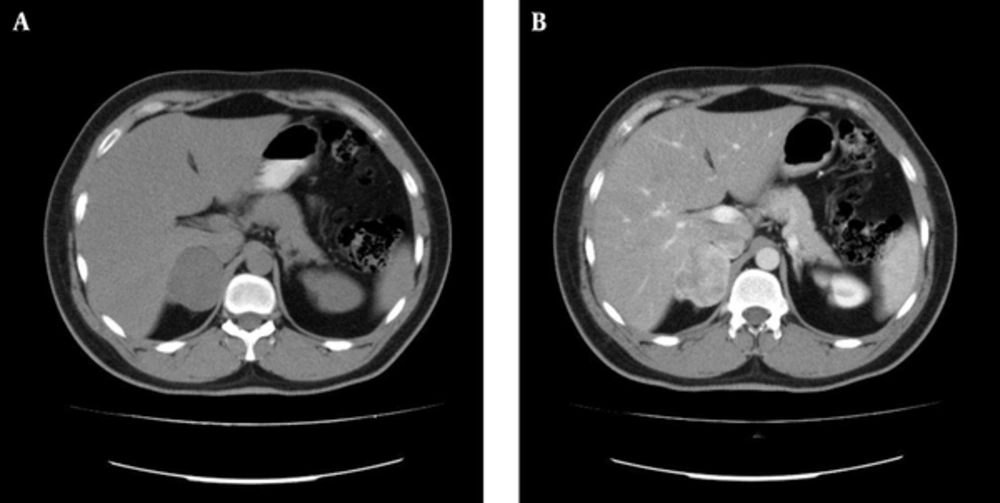
A multi-slice computed tomography scan of the abdomen revealed a mass measured 47 × 42 mm in the right adrenal gland. The mass was irregular and hyperdense (hounsfield units = 28) and had moderate inhomogeneous contrast uptake. Although these findings suggested a pheochromocytoma, they were also compatible with an adrenal malignancy (Figure 1).
Blood concentration of cortisol and dehydroepiandrosterone sulfate were not significantly elevated. The concentration of aldosterone was 11 ng/dL (normal range: 1 - 21) and plasma renin concentration was 9.2 mcU/mL (normal range: 3.3 - 41). Twenty-four-hour urine studies included vanillylmandelic acid 10.7 mg (normal range: 2 - 12 mg/24 hours), metanephrine 116 mcg (normal: < 350 mcg/24 hours) and normetanephrine 594 mcg (normal: < 600 mcg/24 hours) which were all found to be normal. Urinary free cortisol was normal. The complete blood count, plasma levels of electrolytes, kidney and liver function, and urinalysis were normal. Blood biochemistry showed a fasting plasma glucose value of 135 mg/dL and glycated hemoglobin 7.8%.
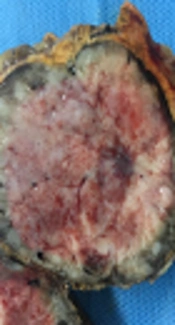
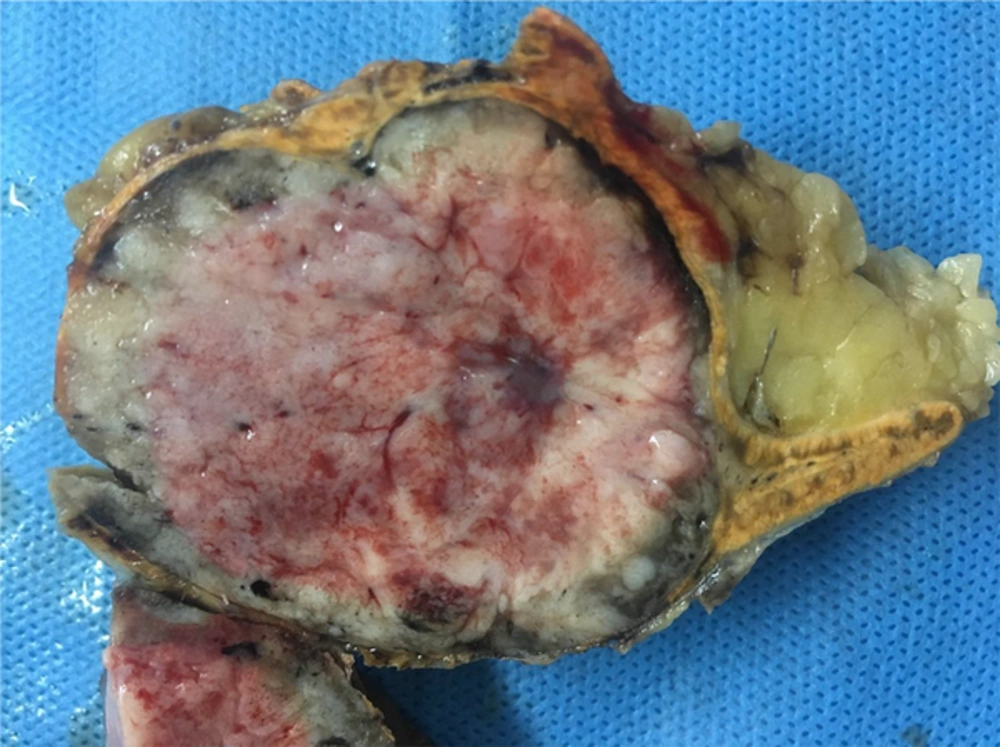
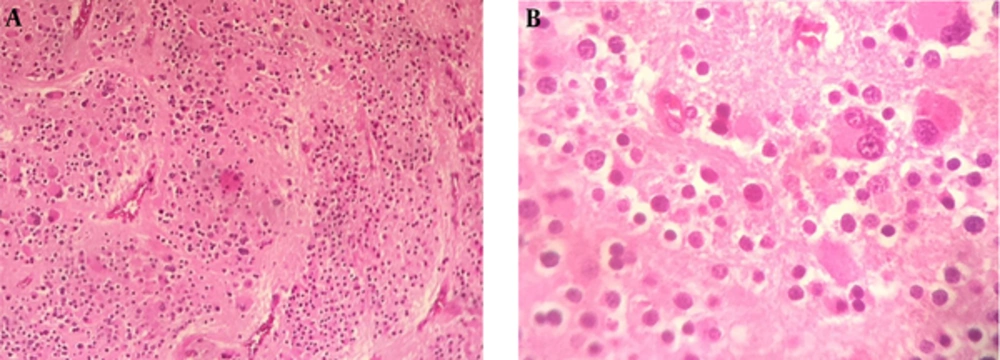
A diagnosis of diabetes and hypertension in this patient and the appearance of the adrenal mass in imaging studies compelled us to remove the tumor surgically because of the chance of an atypical pheochromocytoma or an adrenal malignancy. Therefore, the necessary preoperative preparation was undertaken. Preoperative α-receptor blockade was achieved with prazosin. An adrenal mass resection was scheduled. The peri-operative and post-operative time periods were uneventful. A resection was performed via a trans-abdominal approach. The tumor was encapsulated. The surgical sample sent for pathological examination was composed of a solid, oval mass, tan-brown in color. The mass was 55 mm in its greater diameter and had a remnant of the adrenal gland in its periphery (Figure 2). The histopathological report described an encapsulated proliferation, which consisted of a tumor of small round blue cells characterized by small uniform hyperchromatic nuclei and scant cytoplasm with a vague lobulated architecture. Nodular aggregates of tumor cells were separated by delicate fibrovascular septa. In some areas, tumor cells were surrounded by ganglioneuromatous-like cells characterized by collections of ganglion cells. Many of these were immature multinucleated cells within a fine fibrillary cobweb-like network (Figure 3). The diagnosis was compatible with a ganglioneuroblastoma of the adrenal gland; the nodular classic type. In immunohistochemical staining, the neoplastic cells were immunoreactive for synaptophysin and chromogranin, while they were negative for S100 and vimentin. These findings were consistent with a nodular ganglioneuroblastoma.
The patient received no chemotherapy or radiation. The MIBG (iodine-123-meta-iodobenzylguanidine) scintigraphy following the surgery was negative. Three months after surgery, an abdominal CT scan revealed no local recurrence or distant metastasis. We will continue following up with the patient every three months.
3. Discussion
A ganglioneuroblastoma is an intermediate tumor that arises from nerve tissues. Sometimes the tumor grows slowly, other times it grows and spreads quickly. The cells of a ganglioneuroblastoma have a dual feature. Some are immature and poorly differentiated, and others are mature ganglion cells, specialized with a distinct form and function. Cell differentiation can indicate the likelihood of a tumor to remain localized or metastasize. The pattern of metastases is regional lymph node, adjacent organ, bone marrow, bone, lymph node, liver, intracranial structures, skin and testis (2%) (2). This is a very rare tumor that occurs in children, with a majority of cases seen in young children aged up to four years. It has an incidence rate of less than five cases per 1,000,000 children (3, 4). Fewer than 50 cases of ganglioneuroblastoma have been reported in adults and to our knowledge, only 19 cases of adrenal ganglioneuroblastoma in adults including the present case have been reported (5-22). In these 18 cases, the mean age at diagnosis was 39 years (range: 20 - 67 years). Males were predominantly affected (12: 6). The mean tumor size was 10.4 cm (range: 5 - 18 cm).
The international neuroblastoma pathology classification defined the histological features and recommended four tumor categories: neuroblastoma, ganglioneuroma, ganglioneuroblastoma-intermixed, ganglioneuroblastoma-nodular. The four categories are divided in two distinct prognostic groups: favorable histology and unfavorable histology (23, 24).
The international classification is currently used for the staging of the disease. Important prognostic factors are age at the diagnosis (children aged < 1 year have the most favorable prognosis), primary site of the tumor (retroperitoneum and adrenal gland tumors have a worse prognosis than mediastinum lesions), histology and stage of disease (25, 26).
Clinical presentations of ganglioneuroblastoma are different. The symptoms are a result of a local mass effect or metastasis to various organs. Some cases, such as our case, may be found incidentally. The utility of urine catecholamines in the diagnosis of ganglioneuroblastoma are limited. They cannot be used to differentiate between ganglioneuroblastoma and pheochromocytoma. In our case, we found the upper limit of urinary catecholamines. For incidentalomas, it is difficult to distinguish ganglioneuroblastoma from other adrenal incidentalomas via imaging (27). As preoperative diagnosis is difficult, diagnosis depends on the postoperative pathological findings. Surgical excision of the localized disease appears to be the only curative treatment of adult ganglioneuroblastomas.
Few data are available regarding treatment of adults with ganglioneuroblastomas. It is not clear whether pediatric chemotherapy regimens are effective for adults. Prognosis of adults also remains unclear because of the limited data. Recurrence of disease has been found to occur mostly within the first two years after surgery. Therefore, attention to the symptoms and signs are necessary. Clinical follow-up and imaging studies including CT scans and MIBG scintigraphy should be performed every three months in the first year, then every six months, within the first two years after surgery. In the present case, complete tumor resection was performed, and there was no evidence of metastasis; therefore, post-operative chemotherapy or radiotherapy is not planned for this patient. However, meticulous follow-up is needed.