1. Background
Stress is an unpleasant condition that perturbs homeostasis of the organism having influences on behavioral, endocrinological, and cellular levels (1). On one hand, the body responds against stress as promoting adaptation to the stressor. One of the key elements in this adaptation process is the reaction of the hypothalamic-pituitary-adrenal (HPA) axis with the consequent secretion of the corticotropin releasing hormone (CRH) from hypothalamus, following to the adrenocorticotropic hormone (ACTH) from pituitary gland. In response to ACTH, corticosterone (CORT) as being glucocorticoid (GC), is secreted from the adrenal cortex (2). The activation of specific receptors in certain tissues by CORT stimulates adaptive feedbacks against stress at metabolic, immunomodulatory, neuromodulatory, and behavioral levels (3). One of these feedbacks countered by the systemic effect of CORT includes a reduction in calcium absorption from the intestine and a reduction in calcium reabsorption in the kidney by enhancing parathyroid hormone secretion (4).
Parathormone/parathyroid hormone (PTH), which is secreted from the four parathyroid glands, is the primary regulator and minute to minute determinant of both extracellular and intracellular calcium homeostasis in the blood (5). The PTH exerts its effects directly in the kidney (tubular reabsorption of calcium) and bone (mobilization and resorption of calcium), and indirectly in the intestine (absorption of calcium) (6). The chief cells in the parathyroid glands detect small fluctuations in the levels of blood ionized calcium and modify the PTH secretion, accordingly. PTH initiates these adaptive responses through the calcium-sensing receptor (CaSR) located at the cell surface of chief cells, thyroid C cells and cells of kidney tubules (7). In addition, the limited number of experimental studies are directed as the point of view to the potential role of PTH on the physical/psychological stress response. One of the early in vivo study demonstrated the role of adrenergic system in regulation of the PTH secretion by increasing the level of iPTH acutely during epinephrine infusion rather than isoproterenol or norepinephrine administration in cows (8). In another research, PTH was considered as a candidate of "stress" hormone when the researchers compared the serum calcium changes upon different concentrations of PTH injections to parathyroidectomized male rats after subjected to confinement/ultra high frequency stress (9). In addition, an increase in the blood level of iPTH with a decrease in the level of major stress hormones such as ACTH and cortisol were noted in rabbits exposed to chronic emotional stress (10). However, such defined studies did not elucidate the underlying molecular mechanisms of the interactions between chronic stress and the PTH level.
In this purpose, we aimed to cross-examine the consequences of restraint stress paradigm, which not only produces psychological stress but also mimics physical stress of humans on PTH secretion mechanisms at behavioral, hormonal, and molecular levels in rats.
2. Methods
2.1. Animals
Adult male Wistar albino rats (n = 42) (230 ± 5 g) were housed with ad libitum food and water under standard, stress-free environmental conditions (21°C; 12 hour light/dark cycle). All procedures were designed in accordance with generally accepted ethical standards for animal experimentation and the guidelines established by the local scientific Ethical Committee of Bezmialem Vakif University (Istanbul, Turkey).
2.2. Restraint Stress
Rats (n = 28) were randomly divided into four groups receiving restraint stress for 7 and 28 days and their control counterparts (n = 7 for each group). Rats in both stress groups received restraint stress in plexiglas semi-circular, well ventilated restrainer tubes (length 25 cm, diameter 7 cm), which restricted lateral, backward, and forward movement of rats, however, it did not prevent breathing during random 3 h of light cycle for 7 and 28 consecutive days (11, 12). Immediately after terminating the stress exposure, animals returned to their home cages. Meanwhile, rats in the both control groups were left undisturbed.
2.3. Behavioral Assessments
A different set of animals (n = 14) including both stress and control groups was used for behavioral experiments at day 7 and 28 to reduce the stress-dependent alterations in the blood levels of studied molecules, which may be caused by behavioral assessments.
Elevated plus maze (EPM): The apparatus was composed of two opposed open arms (50 × 10 cm) and two opposed closed arms (50 × 10 × 40 cm) connected by a central platform (5 × 5 cm) positioned 50 cm above the ground. Each animal was placed on the center zone towards one of the open arms and allowed 5 minutes of free exploration. Before each test, the arms were cleaned with 70% of ethanol. The anxiety of animals was calculated according to the time spent in open and enclosed arms during 5 minutes intervals. Percentage of time spent in open arms [%OAT = time in “open arm” / (time in “open arm” + time in “closed arm”) × 100] was calculated considering an index of anxiety (13).
Tail suspension test (TST): The apparatus consisted of a horizontal bar elevated 50 cm above the ground. Each rat was suspended by firming the tail to the bar by wrapping noninvasive adhesive tape. The time spent for immobile posture during a 5-minute testing period was measured. The test was performed by observers who were blinded to the groups (14).
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
Blood samples from the jugular vein of all rats were collected into EDTA containing tubes immediately after stress sessions. To determine the effects of acute stress (3 hours) and chronic stress (3 hour/day for 28 days) on the levels of studied hormones, we collected blood sample on the 1st, 7th, 21st, and 28th days of stress administration. Blood plasma was obtained by centrifugation and stored at -80°C. Plasma levels of adrenocorticotrophic hormone (ACTH) (Intra assay CV%: 10 sensitivity: 2.49 ng/L), corticosterone (CORT) (Intra assay CV%: 10 sensitivity: 2.51 ng/mL), and intact parathormone (iPTH) (Intra assay CV%: 10 sensitivity: 0.51 ng/L) in both stress and control groups were measured using commercially available ELISA kits (Shanghai YeHua Biological Technology Co., Ltd., China), according to the manufacturer’s instructions (14).
2.5. Western Blotting
Frozen kidney and total thyroid gland tissues obtained from all the rats were homogenized with RIPA lysis buffer in the presence of protease and phosphatase inhibitor cocktail. Protein concentrations were determined by BCA assay kit in Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific; Paisley, England). Equal amounts of proteins were separated by sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and were subsequently transferred to a PVDF membrane. Afterwards, the blots were incubated with the primary antibodies anti-PTH/PTHrP-R (PTHR1) (Santa Cruz), anti-CaSR (Thermo Scientific), anti-GR (Thermo Scientific), anti-β-tubulin (Thermo Scientific), and anti-β-actin (Santa Cruz). The blots were then exposed to chemiluminescence solution for visualization of the specific binding. Densitometric quantifications of the protein bands were done using ImageJ analysis system (NIH, Bethesda, USA). Results were normalized against the β-actin or β-tubulin expression in each group (15).
2.6. Statistical Analysis
Statistical analyses of differences among the groups were determined using the Student’s t-test for both behavioral and molecular data (SPSS for Windows, version 18.0, Chicago, IL, USA). The body weights of animals were analyzed by repeated measure of ANOVA. Pearson’s correlation test was also applied to determine the relation between the hormone levels. The results are expressed as mean ± standard error of mean (SEM). Differences were considered as significant at P ≤ 0.05.
3. Results
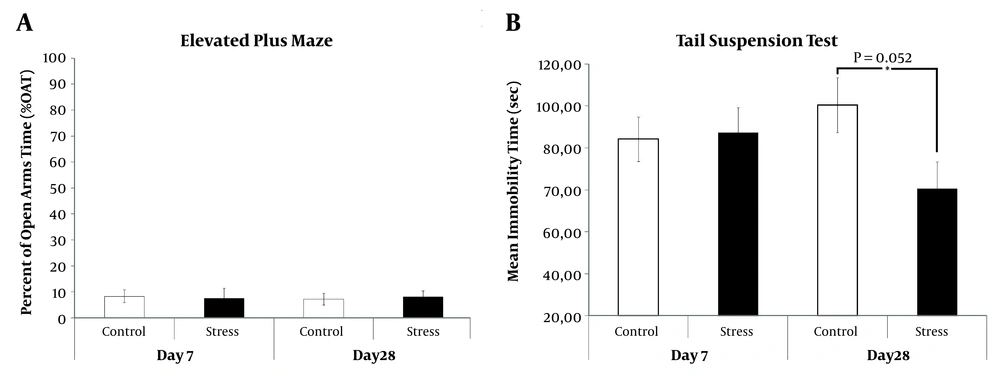
In anxiety-like behavior, which was assessed by EPM, the more time spent in the open arms of the maze was considered as less in the level of anxiety. In the present study, no significant difference in the percentage of time spent in the open arm (%OAT) was detected among groups (Figure 1A). In the depression-like behavior, which was evaluated by the TST, there was a significant decrease in the duration of immobility, which is a state of giving up and despair, at 28-day-CRS received rats as compared to unstressed ones (P = 0.052) (Figure 1B).
Anxiety/depression like behaviors of 7- and 28-day-CRS received rats were tested by EPM and TST, respectively. (A) Percent of open arms time (%OAT) which were recorded during 5 minutes. (B) Total duration of immobility was recorded for 5 minutes. *P < 0.05. Results are expressed as mean and standard error of mean (SEM).
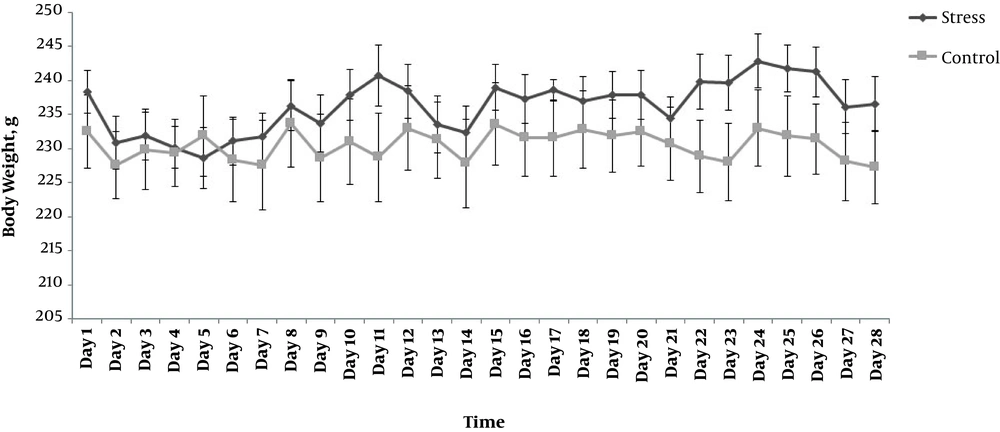
During the whole experiment, all the animals were weighted and their weight gain for each day were presented in the Figure 2. According to the repeated measure ANOVA analyses, there was a significant day effect (F(27,324) = 6.847, P ≤ 0.001) and day X group interaction (F(27,324) = 3.863, P ≤ 0.001), which means that, at some time, the stressed rats gained weight differently from controls. However, there was an insignificant treatment effect between stress and control groups (F(1,12) = 6.847, P = 0.340). Moreover, the analysis, day-by-day (independent t-test), showed no difference between the groups (P > 0.05).
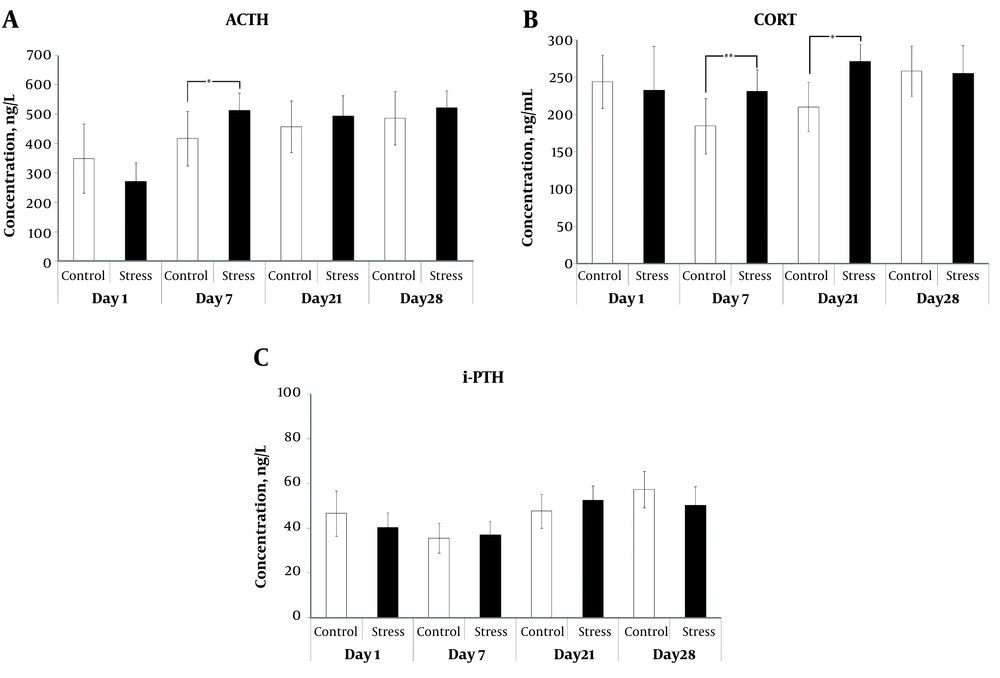
Stress induced changes in the levels of plasma ACTH, CORT, and iPTH were measured for each sampling day (1, 7, 21, and 28) by ELISA method (Figure 3). At day 7, CRS induction significantly increased the plasma ACTH levels as compared to the control counterparts (P = 0.034) (Figure 3A). CRS had also marked elevation in the CORT concentrations at day 7 and 21 as compared to their controls (P = 0.010 and P = 0.016, respectively) (Figure 3B). In the present study, we did not observe any significant change in the iPTH level between the stressed and the control animals at any sampling time (Figure 3C). However, Pearson’s correlation test showed a significant negative correlation between the levels of iPTH and CORT (P = 0.002) after acute stress (Table 1). In addition, both acute stress and CRS (day 7 and 21) disrupted the significant correlation between the levels of CORT and ACTH, which was observed in the control animals (P = 0.005 at day 1, P = 0.028 at day 7, and P = 0.001 at day 21) (Table 1).
| Time | Control Group (N = 7) | Stress Group(N = 7) | ||
|---|---|---|---|---|
| r | p | r | p | |
| Day 1 | ||||
| iPTH correlations with | ||||
| ACTH (ng/L) | 0.292 | 0.332 | 0.243 | 0.424 |
| CORT (ng/mL) | 0.353 | 0.237 | -0.771* | 0.002 |
| ACTH correlations with | ||||
| iPTH (ng/L) | 0.292 | 0.332 | 0.243 | 0.424 |
| CORT (ng/mL) | 0.721* | 0.005 | -0.479 | 0.097 |
| Day 7 | ||||
| iPTH correlations with | ||||
| ACTH (ng/L) | 0.382 | 0.198 | -0.190 | 0.554 |
| CORT (ng/mL) | 0.221 | 0.469 | 0.142 | 0.659 |
| ACTH correlations with | ||||
| iPTH (ng/L) | 0.382 | 0.198 | -0.190 | 0.554 |
| CORT (ng/mL) | 0.585* | 0.028 | 0.248 | 0.438 |
| Day 21 | ||||
| iPTH correlations with | ||||
| ACTH (ng/L) | -0.313 | 0.495 | 0.539 | 0.270 |
| CORT (ng/mL) | -0.295 | 0.521 | -0.080 | 0.880 |
| ACTH correlations with | ||||
| iPTH (ng/L) | -0.313 | 0.495 | 0.539 | 0.270 |
| CORT (ng/mL) | 0.958* | 0.001 | 0.258 | 0.576 |
| Day 28 | ||||
| iPTH correlations with | ||||
| ACTH (ng/L) | 0.103 | 0.846 | 0.537 | 0.214 |
| CORT (ng/mL) | 0.445 | 0.377 | 0.080 | 0.865 |
| ACTH correlations with | ||||
| iPTH (ng/L) | 0.103 | 0.846 | 0.537 | 0.214 |
| CORT (ng/mL) | -0.053 | 0.920 | 0.597 | 0.157 |
Abbreviations: ACTH, adrenocorticotropic hormone; CORT, corticosterone; i-PTH, intact parathormone.
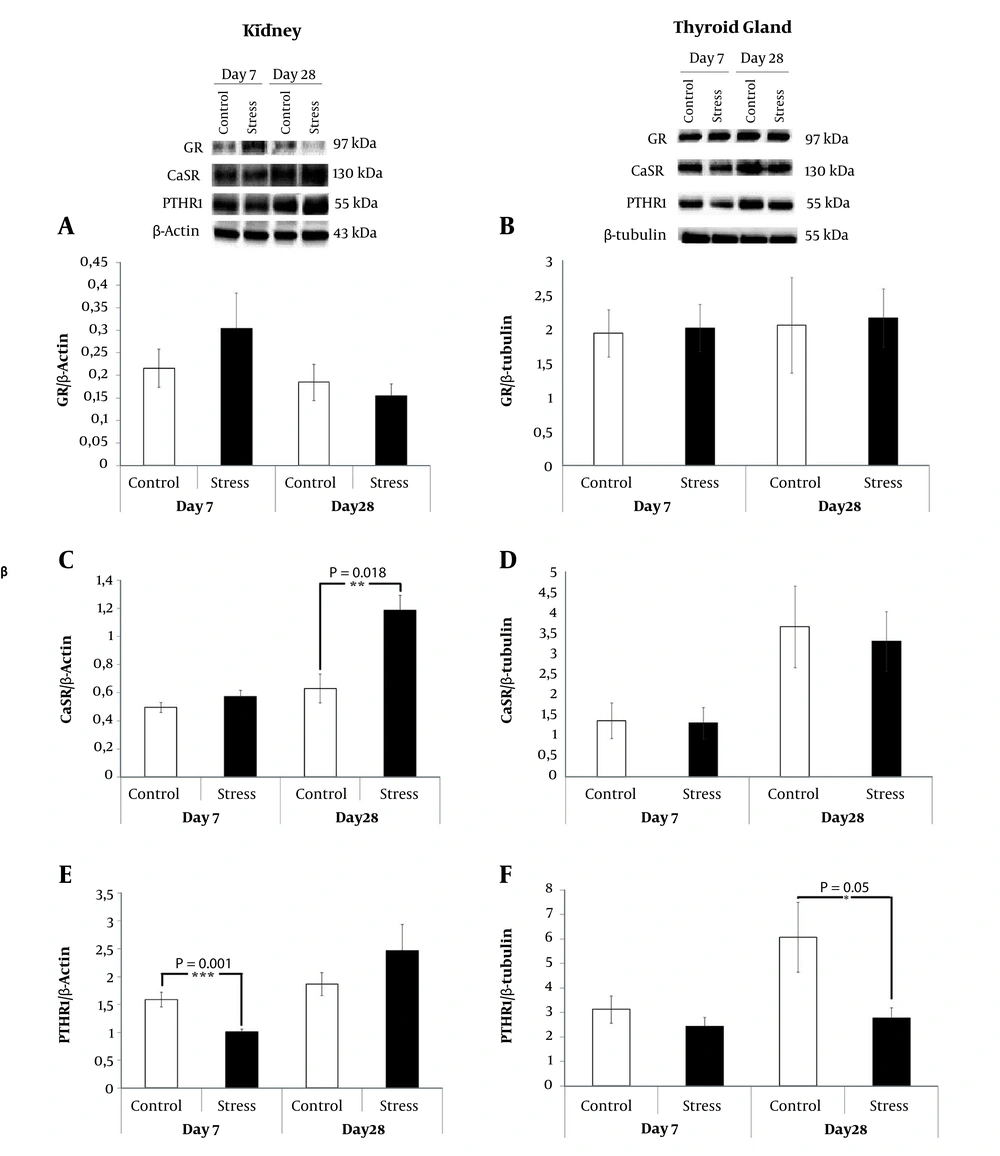
Expression of receptor proteins having a possible role in the stress-induced parathormone secretion were evaluated in the kidney and the total thyroid gland tissues by Western blotting (Figure 4). In the kidney, CRS slightly increased the GR expression only upon 7-day-CRS exposure without reaching the accepted level of significance (P > 0.05) (Figure 4A). The stress-related CaSR expression seemed to be detectable in response to the chronic stress only after 28 days as compared to the control rats in the kidney tissue (P = 0.018) (Figure 4C). In the kidney, a significant decrease was recorded in the expression of PTHR1 upon 7-day-CRS (P = 0.001) (Figure 4E). In the parathyroid gland, we also noted a significant decrease in the expression of PTHR1 in the 28-day-CRS received rats compared to the control rats (P = 0.05) (Figure 4F).
The expressions of renal proteins ((A) GR (C) CaSR (E) PTHR1) with respect to the expression of β-actin in response to 7 and 28 days CRS were analyzed by Western blotting. The expressions of total thyroid gland proteins ((B) GR (D) CaSR (F) PTHR1) with respect to the expression of β-tubulin in response to 7 and 28 days CRS were analyzed by Western blotting. *P < 0.05. Results are expressed as mean and standard error of mean (SEM).
4. Discussion
In the present study, for the first time, we demonstrated the cross examination of the iPTH levels in blood and its molecular targets in kidney and thyroid gland tissues of restraint stress received rats. Restraint stress is the most commonly applied stress model among the laboratory animals due to adequately mimicking both physical and psychological stress in humans (12). Herein, we recorded no effect of restraint stress on the body weight gain of stressed-animals compared to the control group. Furthermore, behavioral alterations were analyzed after 7- and 28-day-CRS exposure according to EPM and TST results. The present results indicated that restraint stress had any effects on both aggressiveness and depression at day 7. Chiba et al. (16), showed that male Wistar rats, having 6 hours restraint stress daily for one week, manifested no elevation in anxiety and depressive-like behaviors obtained from EPM and forced swim test (FST) assessments, which confirms our results. This possibility was also supported by our observations that the amount of defecation, struggle, and vocalization of stressed rats while being placed into the restrainers gradually decreased after one week of CRS. Besides, sustained 28-day-CRS decreased depression-like behaviors in the stress group compared to their controls. One explanation to this may be due to habituation as an adverse consequence of repeated chronic restraint stress or neuroendocrine adaptation of body to cope with stress (12, 17). This antidepressant-like adaptation as a resilience occurs due to the necessity in future stress challenges. It was noted that stress-induced HPA activation can promote resilience (18). Previous studies showed that many signaling pathways have been shown to be engaged in this behavior, including the mammalian target of rapamycin (mTOR) pathway (19) and glutamatergic signaling pathway (18).
The major defense in rodents against any kind of stress is the secretion of GCs that in turn result in CRH secretion from adenohypophysis, which sequentially triggers ACTH and CORT release from adrenal cortex (3). Although there has been a growing evidence that acute restraint stress causes an increase in the activity of HPA axis, we observed no significant change in the levels of both ACTH and CORT after the first 3 hours of restraint stress, which might be derived from uncontrolled stress conditions in the control group during blood sampling. Another reason for unchanged ACTH and CORT levels after acute restraint stress administration in contrast to the literature might also be a result of the methodological differences and the time of blood sampling (20-22). Along with these, the level of iPTH was also not affected from acute restraint stress. The activity of HPA axis was increased at day 7 and 21 of CRS, as shown by increments in the plasma levels of ACTH and CORT compared to the unstressed rats, indicating that our repeated stress model is sufficiently potent to induce a psychological chronic stress at the hormonal level. On the other hand, this chronic stress model disrupted the positive correlation between ACTH and CORT levels, which was observed in the control group. During application of stress, alterations in the plasma GCs levels inhibit HPA axis as a negative feedback for adaptation of HPA axis responsiveness to new stressors (23). For circulating iPTH level, we only obtained a significant negative correlation between plasma CORT and iPTH levels during acute stress meaning that an increase in CORT, as a response of stress induction, causes a decrease in iPTH level. In one of the previous studies, a similar negative correlation in the levels of parathormone related protein (PTHrP) and CORT was evidenced in the animals exposed to cold restraint stress (24). It is well known that there is an interaction between CORT and PTH levels in the regulation of the viability and differentiation of both chondrocytes and bone cells during developmental stages (25). Moreover, it was also shown that CORT and PTH work against each other in bone remodeling after unwanted situations (26). These knowledge supported our result that CORT exerts its function on iPTH levels to adapt the restraint stress response by decreasing its level.
Due to the fact that studies, on the findings association between stress and PTH secretion, have not extended beyond acute adrenergic stimuli or acute restraint stress response to PTH and calcium measurements, respectively (8, 9). For the first time, we tried to elucidate the molecular basis of afore-mentioned behavioral and hormonal changes with respect to chronic restraint stress. For this purpose, we examined the expressions of GR, CaSR, and PTHR by comparing 7 and 28-days CRS received rats to their controls in the target tissues. GCs autoregulate GR expression, which can be either positive or negative by modulating the cellular sensitivity to the hormone, to control alterations in the homeostatic environment as a result of stress in a variety of tissues (27). In the present study, the decrease in the depression-like behavior might be illustration of a negative feedback autoregulation of 28-day-CRS induced GR expressions in the kidney.
CaSR belongs to a family of G protein-coupled receptor, and it is expressed most abundantly in kidney and parathyroid glands being responsible for the calcium-dependent inhibition of the PTH secretion (28). To enlighten the mechanism, whether stress altered PTH secretion have an effect on target tissues, we further analyzed the CaSR expression and found a significant increase in the renal CaSR expression in the 28-day-CRS received rats. There are several factors that upregulate the expression of the CaSR, including calcium (29), vitamin D (30), and the cytokines (31). In addition, chronic intermitted stress increased the acute induction of the pro-inflammatory cytokines and the chemokines in plasma (32). Therefore, we propose that the upregulation of CaSR in the kidney may be related with stress-induced increase in the level of blood cytokines.
PTH normally regulates serum calcium levels by binding and activating type 1 PTH receptor 1 (PTHR1) in the bone and kidney (33). In the current study, we showed a stress-related decrease in the PTHR1 expression in the kidney of the 7-day-CRS received rats and in the total thyroid gland of the 28-day-CRS received rats. These decreases in the PTHR1 might be signs for the disease progression in the stress conditions, as it is understood from the study, which showed the correlation between downregulation of PTH1R and the pathogenesis of human end-stage bladder disease (34).
The absence of comparison of all the molecular and behavioral parameters for both acute and chronic stress might be considered as one of the limitations of the present study. Another draw-back of the current study is the absence of significant differences in the levels of iPTH in all studied time points due to high standard deviations that may be resulted from insufficient sample size due to ethical considerations. On the other side, one of the most powerful strengths of this study is being the first research on the association between PTH plasma concentration and the expression of its molecular targets of the restraint stress in rats.
4.1. Conclusions
The present findings showed that chronic restraint stress has a remarkable effect on the expression of PTH1R rather than iPTH concentration in the blood. Therefore, this study may contribute a new dimension to the stress-related literature, which has had over the past decade harbored limited evidences about stress and stress-related molecular mechanisms on PTH secretion. However, the underlying molecular mechanisms of PTH secretion, in response to stress, must be precisely elucidated in future in vivo studies.