1. Background
Individuals with type 2 diabetes (T2D) are more vulnerable to both short- and long-term complications often leading to premature death and reduced life expectancy (1, 2). The risk of cardiovascular disease (CVD) and stroke in people with T2D is 2 - 4 and 1.5 - 5 times that of the general population, respectively. CVD in the T2D population accounts for almost 70% of deaths and stroke has higher severity and mortality among this population (3-5). Almost 20% of deaths in patients with T2D are attributed to cerebrovascular accidents (CVA) (6). There is increasing biological and epidemiological evidence of the association between diabetes (mainly type 2) and the increased risk of cancer in advanced stages with high levels of mortality (7-9). Foot infections and ulcers are important causes of hospitalization and the leading causes of morbidity in T2D (10). The prevalence of renal disorders in individuals with T2D is estimated to be 17 times that in the general population. These include microvascular complications such as diabetic nephropathy that is the main cause of the end-stage renal disease (ESRD) worldwide (5, 11).
Generally, patients with T2D may experience different events and the corresponding risk factors among them are usually evaluated using logistic regression. However, this approach overlooks time to occurrence of the event of interest. From this viewpoint, time-to-event or survival analysis methods are preferred. However, when more than a single event is evaluated, simple methods such as Cox regression could give inaccurate estimates, as they ignore associations between the events that idiomatically compete against one another to occur earlier. In an effort to settle this issue, competing risks models have been suggested.
Despite the popularity of competing risks modeling in various disciplines of medicine, studies using this approach in the diabetes context are not frequent (12-14). In a recent study, the risk of death from CVD, cancer, and other (non-cardiovascular-non-cancer) causes has been evaluated without evaluating risk factors of each death type (2). Nevertheless, there are studies in patients with T2D that examined risk factors for a particular cause (15, 16).
2. Objectives
The current study aimed to examine the cause-specific risk factors for each cause of death (CVD, CVA, cancer, and foot infection/diabetic nephropathy) in T2D using competing risks analysis.
3. Methods
3.1. Study Population
We retrieved the data of 2638 people with T2D (1110 men and 1528 women) from the database of the Isfahan Endocrine and Metabolism Research Center from 1992 to 2004 with a median follow-up of 60 months. All patients aged ≥ 35 were included in the analysis. Type 1 diabetes, death from causes other than those under study, and missing data were the criteria for exclusion from the analysis.
Of the 2638 people with T2D, 395 (15%) suffered from various risk events including death from CVD, CVA, cancer, and foot infections/diabetic nephropathy. There were 215 patients (8.2%) who died of myocardial infarction, 89 (3.4%) of stroke, 54 (2%) of cancer, and 37 (1.4%) of foot infections/diabetic nephropathy; thus, 2243 (85%) patients did not experience any of the final points considered as censored in this study.
All cases of coronary heart disease, congestive heart failure, rheumatic heart disease, cardiomyopathy, and other heart diseases were considered as CVD. Ischemic stroke and hemorrhagic stroke were included in the CVA definition. Individuals with a history of any events under study at the baseline were excluded from the study.
3.2. Measurements and Outcomes
Demographic information and duration of diabetes were recorded by a trained interviewer using a standardized questionnaire. An inflexible bar was used to measure the height of participants standing straight against the wall. A digital scale was used to measure weight rounded to the nearest 100 g. Measurements were done in light clothing taking socks and shoes off. Blood pressure (BP) was measured after 15-minutes rest in the sitting position with appropriately sized cuffs using a standardized mercury sphygmomanometer on the right arm at two time-points of the 5-minutes interval. The mean of these measures was recorded as BP if the differences between the two measures of systolic and diastolic BP were less than 10. Otherwise, a third measurement was done and the average was recorded. Patients with a history of antihypertensive drug use, SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg were considered hypertensive (17, 18).
Fasting blood sugar (FBS), triglyceride (TG), and cholesterol levels were evaluated using blood tests. Those with FBS ≥ 126 mg/dL were considered hyperglycemic. TG and cholesterol levels were categorized into two groups in 200 and 240 mg/dL, respectively. Most T2D patients are exposed to various risks after the age of 65. Therefore, we classified patients into two groups of ≤ 65 and > 65 years in the analysis. Detailed information on measurements and procedures is available elsewhere (15).
The composite outcome was the time from diabetes diagnosis to death from CVD, CVA, cancer, or foot infection/diabetic nephropathy. Patients who lost the follow-up or experienced none of these endpoints in the study period were considered as censored in the analysis. Hence, the survival time was the time from diagnosis of diabetes to experiencing the event, last visit, or end of the study.
3.3. Statistical Analysis
We used two main approaches introduced in the context of competing risks models. Each method answers a different research question. The following is a brief review of these methods. More details can be found elsewhere (19, 20).
The first model for analyzing competing risks data is the cause-specific hazard (CSH) model that is a generalization of the traditional Cox model to compensate for the overestimation bias (19). In this model, the effect of covariates on the risk of experiencing each of competing events at any specific time is evaluated over those subjects who have experienced none of the events up to that time. That is, the CSH model gives an instant rate of occurrence of each event in subjects who have not yet experienced either event. The interpretation of associations is through the well-known hazard ratio (HR) in a similar way to the Cox regression. The second method is the subdistribution hazards (SDH) model introduced by Fine and Gray in 1999 (21). In this approach, instead of the modeling hazard function, cumulative incidence function (CIF) is modeled. Thus, the SDH model transfers a different concept. At any time, the effect of covariates on the risk of experiencing each of competing events is evaluated over all subjects who have not have that event until that time. Here, at any time, the risk set includes all subjects in the study except for those who have experienced the event under consideration.
The CSH and SDH models use different methods and answer different types of questions, as well. In brief, the SDH model is used when the researcher aims to assess the overall impact of a covariate on the incidence of each event, i.e. the estimation of actual risks and prognosis, or risk prediction for a specific person. The CSH model is more concerned with studying the etiology of diseases (19). Nevertheless, less computational demand and simple interpretations are the merits of the CSH model. The SDH model not only informs patients about the risks they face in certain situations, but also can guide clinicians in assigning a specific treatment regimen to a patient (22).
Cumulative incidence of an event is often of interest and is frequently reported in medical research. The graphical display of the CIF (i.e., failure probabilities) over time is intuitive and appealing. In the presence of competing risks, a class of tests has been proposed in the literature for comparing the cumulative incidence curves of a particular type of failure among different groups (23).
We included the following predictors altogether in one-step analysis: duration of diabetes, blood pressure, hyperglycemia, triglyceride, and hypercholesterolemia. The results were adjusted for age, sex, and BMI.
Statistical analysis was carried out using R package cmprsk (http://www.r-project.org). Descriptive statistics were presented as No. (%) and mean ± SD. The statistical significance level was set at 0.05.
4. Results
The baseline characteristics of the total sample of 2638 subjects included in the final analysis are shown in Table 1. Briefly, 57.9% of the patients were female and the mean age of the patients was 55.16 ± 9.86 years (min = 35, max = 92). Of the total sample, 215 (8.2%) patients died from CVD, 89 (3.4%) from CVA, 54 (2%) from cancer, and 37 (1.4%) from foot infection/diabetic nephropathy that accounted for 54.4%, 22.5%, 13.7%, and 9.4% of the total deaths in this population, respectively.
| Variable | Total (N = 2638) | CVD (N = 215) | CVA (N = 89) | Cancer (N = 54) | Foot Infection/Diabetic Nephropathy (N = 37) |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 1528 (57.9) | 81 (37.7) | 37 (41.6) | 22 (40.7) | 13 (35.1) |
| Male | 1110 (42.1) | 134 (62.3) | 52 (58.4) | 32 (59.3) | 24 (64.9) |
| Age, y | |||||
| ≤ 65 | 2272 (86.1) | 159 (74.0) | 56 (62.9) | 40 (74.1) | 28 (75.7) |
| > 65 | 366 (13.9) | 56 (26.0) | 33 (37.1) | 14 (25.9) | 9 (24.3) |
| BMI, kg/m2 | |||||
| ≤ 25 | 718 (27.2) | 88 (40.9) | 38 (42.7) | 20 (37.0) | 18 (48.6) |
| > 25 | 1920 (72.8) | 127 (59.1) | 51 (57.3) | 34 (63.0) | 19 (51.4) |
| Diabetes duration, y | |||||
| ≤ 10 | 2072 (78.5) | 136 (63.3) | 52 (58.4) | 38 (70.4) | 20 (54.1) |
| 10 - 20 | 474 (18.0) | 60 (27.9) | 32 (36.0) | 10 (18.5) | 13 (35.1) |
| > 20 | 92 (3.5) | 19 (8.8) | 5 (5.6) | 6 (11.1) | 4 (10.8) |
| Hypertension | |||||
| Yes | 1319 (50.0) | 145 (67.4) | 68 (76.4) | 33 (61.1) | 25 (67.6) |
| No | 1319 (50.0) | 70 (32.6) | 21 (23.6) | 21 (38.9) | 12 (32.4) |
| FBS, mg/dL | |||||
| ≤ 126 | 600 (22.7) | 33 (15.3) | 5 (5.6) | 8 (14.8) | 2 (5.4) |
| > 126 | 2038 (77.3) | 182 (84.7) | 84 (94.4) | 46 (85.2) | 35 (94.6) |
| Triglyceride, mg/dL | |||||
| ≤ 200 | 1677(63.6) | 121 (56.3) | 51 (57.3) | 43 (79.6) | 17 (45.9) |
| > 200 | 961 (36.4) | 94 (43.7) | 38 (42.7) | 11 (20.4) | 20 (54.1) |
| Cholesterol, mg/dL | |||||
| ≤ 240 | 2000 (75.8) | 141 (65.6) | 65 (73.0) | 46 (85.2) | 22 (59.5) |
| > 240 | 638 (24.2) | 74 (34.4) | 24 (27.0) | 8 (14.8) | 15 (40.5) |
Abbreviations: BMI, body mass index; CVA, cerebrovascular accident; CVD, cardiovascular disease; FBS, fasting blood sugar.
aValues are expressed as No. (%).
The HR and corresponding 95% confidence intervals (CI) after adjusting for sex, age, and BMI are shown in Table 2. The risk of death from CVD increased with hypertension (HR = 1.83, 95% CI: 1.37 - 2.46), hypercholesterolemia (HR = 1.58, 95% CI: 1.17 - 2.14), and diabetes duration. Hypertension (HR = 2.76, 95% CI: 1.67 - 4.55) and hyperglycemia (HR = 4.34, 95% CI: 1.75 - 10.79) were associated with higher risks of death from CVA and the risk in patients with diabetes duration of 10 - 20 years was higher than the risk of other patients. Diabetes duration of longer than 20 years was associated with a higher risk of death from cancer (HR = 2.65, 95% CI: 1.05 - 6.68). In addition, patients with diabetes duration of 10-20 years were at higher risk of death from foot infection/diabetic nephropathy than the patients of other ages (HR = 2.20, 95% CI: 1.07 - 4.50). Almost similar results were seen in both analyses with stronger associations in the CSH models as expected. However, the factors associated with the last two death causes were found to be significant only in the CSH model.
| Death Causes and Predictors | Model | |
|---|---|---|
| Cause-Specific Hazard Model HR (95% CI) | Sub-Distribution Hazard Model HR (95% CI) | |
| Death from CVD | ||
| Diabetes duration, yb | ||
| 10 - 20 | 1.67 (1.22 - 2.29)c | 1.61 (1.17 - 2.22)c |
| > 20 | 2.34 (1.40 - 3.91)c | 2.15 (1.28 - 3.62)c |
| Hypertension (yes) | 1.83 (1.37 - 2.46)c | 1.76 (1.31 - 2.36)c |
| Hyperglycemia (yes) | 1.38 (0.94 - 2.02) | 1.33 (0.90 - 1.95) |
| Hypertriglyceridemia (> 200 mg/dL) | 1.23 (0.92 - 1.64) | 1.23 (0.93 - 1.64) |
| Hypercholesterolemia (> 240 mg/dL) | 1.58 (1.17 - 2.14)c | 1.59 (1.17 - 2.16)c |
| Death from CVA | ||
| Diabetes duration, yb | ||
| 10 - 20 | 2.07 (1.32 - 3.26)c | 1.98 (1.25 - 3.12)c |
| > 20 | 1.20 (0.46 - 3.11) | 1.05 (0.40 - 2.75) |
| Hypertension (yes) | 2.76 (1.67 - 4.55)c | 2.59 (1.58 - 4.25)c |
| Hyperglycemia (yes) | 4.34 (1.75 - 10.79)c | 4.22 (1.68 - 10.59)c |
| Hypertriglyceridemia (> 200 mg/dL) | 1.28 (0.82 - 2.02) | 1.26 (0.79 - 2.00) |
| Hypercholesterolemia (> 240 mg/dL) | 0.93 (0.56 - 1.54) | 0.92 (0.54 - 1.57) |
| Death from Cancer | ||
| Diabetes duration, yb | ||
| 10 - 20 | 1.05 (0.51 - 2.14) | 1.01 (0.48 - 2.11) |
| > 20 | 2.65 (1.05 - 6.68)c | 2.39 (0.85 - 6.71) |
| Hypertension (yes) | 1.52 (0.86 - 2.68) | 1.45 (0.82 - 2.56) |
| Hyperglycemia (yes) | 1.82 (0.85 - 3.90) | 1.80 (0.83 - 3.90) |
| Hypertriglyceridemia (> 200 mg/dL) | 0.5 (0.25 - 1.01) | 0.49 (0.24 - 1.03) |
| Hypercholesterolemia (> 240 mg/dL) | 0.67 (0.30 - 1.47) | 0.65 (0.28 - 1.54) |
| Death from Foot Infection/Diabetic Nephropathy | ||
| Diabetes duration, yb | ||
| 10 - 20 | 2.20 (1.07 - 4.50)c | 2.07 (0.48 - 4.32) |
| > 20 | 2.87 (0.91 - 8.96) | 2.61 (0.38 - 8.32) |
| Hypertension (yes) | 1.64 (0.80 - 3.34) | 1.55 (0.74 - 3.26) |
| Hyperglycemia (yes) | 3.82 (0.90 - 16.09) | 3.79 (0.87 - 16.56) |
| Hypertriglyceridemia (> 200 mg/dL) | 1.70 (0.85 - 3.38) | 1.68 (0.85 - 3.29) |
| Hypercholesterolemia (> 240 mg/dL) | 1.76 (0.87 - 3.57) | 1.76 (0.90 - 3.44) |
Abbreviations: CVA, cerebrovascular accident; CVD, cardiovascular disease; HR, hazard ratio.
aResults are adjusted for age, sex, and BMI.
bDiabetes duration of ≤ 10 years as the reference category
cSignificant factors.
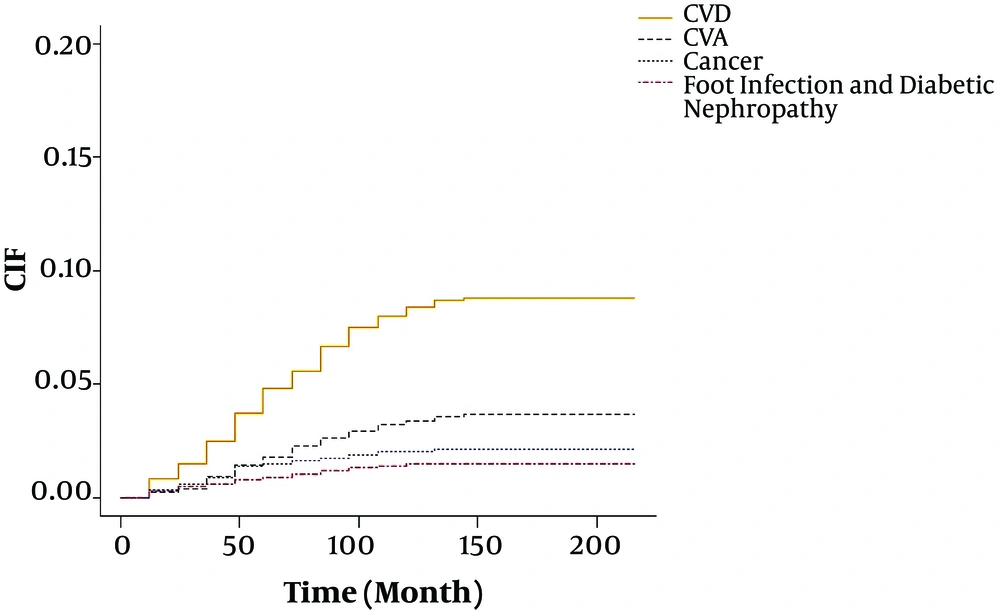
According to the estimate of the cumulative incidence for competing events at various time points shown in Table 3, the probability of death from CVD, CVA, cancer, and foot infection/diabetic nephropathy was respectively 8.4%, 3.3%, 2.0%, and 1.4% after 120 months and 8.7%, 3.6%, 2.1%, and 1.4% after 216 months.
| Type of Event | ||||
|---|---|---|---|---|
| Time, mo | Cardiovascular Disease | Cerebrovascular Accident | Cancer | Foot Infection/Diabetic Nephropathy |
| 24 | 0.014 (5.55e - 06) | 0.003 (1.44e - 06) | 0.006 (2.30e - 06) | 0.004 (1.86e - 06) |
| 48 | 0.037 (1.36e - 05) | 0.014 (5.35e - 06) | 0.013 (5.19e - 06) | 0.008 (3.03e - 06) |
| 72 | 0.055 (2.08e - 05) | 0.022 (8.80e - 06) | 0.016 (6.24e - 06) | 0.010 (4.09e - 06) |
| 96 | 0.074 (2.80e - 05) | 0.029 (1.15e - 05) | 0.018 (7.30e - 06) | 0.013 (5.34e - 06) |
| 120 | 0.084 (3.15e - 05) | 0.033 (1.33e - 05) | 0.020 (8.03e - 06) | 0.014 (5.91e - 06) |
| 168 | 0.087 (3.29e - 05) | 0.036 (1.45e - 05) | 0.021 (8.42e - 06) | 0.014 (5.91e - 06) |
| 192 | 0.087 (3.29e - 05) | 0.036 (1.45e - 05) | 0.021 (8.42e - 06) | 0.014 (5.91e - 06) |
| 216 | 0.087 (3.29e - 05) | 0.036 (1.45e - 05) | 0.021 (8.42e - 06) | 0.014 (5.91e - 06) |
Figure 1 summarizes the cumulative incidence estimates for all the outcomes taking competing risks into accounts. The probability of death due to CVD, CVA, cancer, and foot infection/diabetic nephropathy was almost the same until the 24th month. However, after month 24, the risk of death from CVD was relatively higher than the risk from other events. In other words, the risk of death from any cause increases over time and patients who develop CVD are at higher risk of death, especially after 24 months.
5. Discussion
In this study, we used CSH and SDH models for modeling competing risks of death and related risk factors in patients with T2D. After adjusting for age, sex, and BMI, hypercholesterolemia and diabetes duration of longer than 10 years were found to be associated with higher risks of death from CVD. Moreover, hypertension, higher FBS levels, and diabetes duration of 10 - 20 years increased the risk of death from CVA. However, the impact of diabetes duration on CVA death risk was not significant for durations of longer than 20 years. This may be due to the small number of patients in this category. Meanwhile, other unknown biological processes may have played a role.
Finally, long diabetes duration significantly affected death from foot infection/diabetic nephropathy. As expected, the HRs estimated by the CSH model were higher than those estimated by the SDH model. The negligence of the association inherently existing between competing events may have influenced the results and interpretation of the prognostic effects of different events.
Our results are in concordance with the findings from previous studies. We found a direct association between hypertension and hypercholesterolemia and the risk of death due to CVD in patients with T2D. It has been reported that higher blood pressure is a strong stimulus for CVD in people with T2D (24-26). Among various risk factors for CVD, the relationship between hypercholesterolemia and CVD has long been recognized (27, 28). An epidemiologic survey demonstrated that elevated levels of total cholesterol and LDL-C were associated with increased risk of coronary heart disease (29). A Finnish study showed that the mortality rate from CVD among people with high levels of cholesterol was five times that in the general population and the reduction of serum cholesterol levels up to 10% could reduce mortality due to CVD up to 30% (30).
The duration of diabetes also increased the risk of death from CVD in patients with T2D. Moreover, T2D was associated with elevated total and coronary heart disease mortality and the longer duration of diabetes was reported to be a strong predictor of death among these patients (31, 32).
It is claimed that CVD is the main cause of death in patients with T2D and the rates of mortality from natural causes rise when the duration of diabetes increases (33).
We found a significant association between blood pressure and fasting blood sugar and the risk of death due to stroke in T2D patients. It has been suggested that these factors increase the risk of stroke even after adjustment for other variables (34, 35). Moreover, our results indicated that the duration of diabetes was positively correlated with stroke. This is in accordance with the findings of previous research where the risk of stroke in T2D patients was reported to be three times that in the general population (36, 37).
The duration of diabetes has been declared to be one of the most important factors in the risk of cancer and death among T2D patients (38). Similarly, diabetes duration predicts the progression of nephropathy in these patients (39, 40). Diabetes duration could also predispose both men and women to diabetic foot ulcers (41, 42). It should be noted that, in general, the estimated covariate effects using the CSH and SDH models may be different (13).
Our study is not free of limitations. First, part of data, such as diabetes duration, relied on self-reports. Furthermore, the sample size was intrinsically low for some risk factors, e.g. long diabetes duration, and outcomes, e.g. death from foot infection/diabetic nephropathy. This led to the low power of the tests for these groups as, despite clinically large estimates of HRs, the associations were not statistically significant. Steady cumulative incidence after about 150 months could be the effect of low sample size, as well.
This study examined the impact of various risk factors on the risk of death from different events in individuals with T2D using competing risks analysis that is a better choice in the presence of multiple possible events. Studies in competing risks setting usually report the results of one analytic method. We implemented both CSH and SDH models to give information for target-specific interventions and attain the comprehensiveness of the study.
5.1. Conclusions
Regardless of the cause, death rates in individuals with T2D increase over time and risk factors have different impacts on death from each cause. This should be acknowledged in risk management in individuals with T2D.