1. Background
Chronic kidney disease (CKD) is a growing public health problem associated with age-related renal function failure (1). Today, the most common causes of CKD are obesity, diabetes and hypertension, all of which are components of the metabolic syndrome (MetS) and risk factors for cardiovascular diseases (2). MetS is a cluster of medical conditions consisting of abdominal obesity, hypertension, dyslipidemia and impaired glucose metabolism (3). While the association of each component of the MetS with the development or progression of CKD has been established, there is substantial discrepancy as to whether MetS is a novel risk factor for CKD (4).
CKD is more likely to develop in patients with MetS, and the risk of CKD increases with the number of MetS components (4). However, it remains unclear whether the clustering of these components can predict the risk of CKD. On the other hand, studies differ in the arrangement of MetS components, which is a stronger predictor of CKD (5). The clinical effects of MetS on the prediction of CKD vary among ethnic groups (6), and understanding metabolic risk factors and predictive values of each component affecting the relationship between CKD and MetS is essential in different ethnic populations.
A limited number of studies have assessed the association of MetS and CKD among the Iranian population (7). While each component alone could increase the risk of CKD, studies have shown that the MetS components act synergistically (8). However, it is not clear how these components collectively are associated to CKD in the Iranian population.
2. Objectives
In this study, we sought to explore the association of MetS with this condition and weigh the probabilities of the MetS components as an additional factor to capture the risk factors for CKD in the Iranian population in southern Iran.
3. Methods
3.1. Study Population
This study was conducted in Shiraz, a main urban area in south of Iran, from November 2013 to September 2014. The study involved a randomly selected community sample of the general population in Shiraz. Multistage weight-based random cluster sampling was used based on home addresses and postal zip codes to draw the samples from all the seven municipality districts of Shiraz city. People aged 18 years and older from the randomly selected addresses were interviewed. Foreign residents, pregnant women or those who had history of delivery in the past six months were excluded. The research protocol was approved by the institutional review board and the Ethics Committee of Shiraz University of Medical Sciences. Written informed consents were obtained from all the participants before entering the study.
Demographic characteristics and medical history such as diabetes mellitus, hypertension, and history of cardiovascular or kidney diseases were recorded. A physician and two nurses performed the physical examinations.
The sample size was calculated according to previous studies and using the following formula:
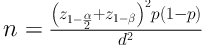
d = 0.03; α = 0.05; z1-α/2 = 1:96; z1-β = 0.80; p = 0.116
Therefore, the standard sample size was calculated at 800 subjects.
3.2. Anthropometric and Physical Measurements
Height and weight were measured by two experienced nurses using calibrated standard scales with participants dressed in light clothing and barefooted. Body mass index (BMI) was obtained by dividing weight in kilograms by the square of height in meters. Waist circumference was measured at the level midway between the 12th rib and the iliac crest using a measuring tape. Blood pressure was recorded in mmHg with a mercury device according to the standardized protocol (9).
3.3. Sample Collection and Biochemical Analysis
After overnight fasting, 10-mL blood samples were taken and centrifuged within 30 minutes of collection and stored at -20°C until further analysis. Blood glucose was measured using a spectrophotometer. Serum total cholesterol, high-density lipoprotein cholesterol (HDL-C) and total glycerol (TG) concentrations were assessed by enzymatic reagents (Biosystems, Barcelona, Spain) with an A-25 BiosystemAutoanalyser. Friedewald equation was used to estimate the low-density lipoprotein (LDL) concentration indirectly from the measured levels of TG, HDL-C and total cholesterol. The intra- and inter-assay coefficients of variation were 0.8% and 3.1% for TC, 0.9% and 2.1% for TG and 2.1% and 3.4% for HDL-C, respectively. Serum creatinine was measured with Jaffe's kinetic method. Intra- and inter-assay coefficients of variation were 2.4% and 3.1%, respectively.
3.4. Definition
CKD Epidemiology Collaboration (CKD-EPI) equation was used to classify the subjects into various CKD stages (10). In stages 1 and 2 with a mild degree of renal impairment, glomerular filtration rates (GFRs) are ≥ 90 and 60 - 89 mL/min/1.73 m2, with albuminuria as a marker of kidney damage. Higher stages are defined only by GFR, which are 73 m2 30 - 59, 15 - 29, and 15 mL/min/1.73 m2 in stages 3, 4 and 5 respectively. In this study, the prevalence of stages 3 to 5 of CKD was defined as an estimated GFR (eGFR) < 60 mL/min/1.73 m2. The demographic and clinical variables were compared between the groups of patients with GFR less than 60 mL/min/1.73 m2 and those without CKD (GFR > 60 mL/min/1.73 m2).
MetS was defined according to the International Diabetes Federation Guideline as being centrally obese with waist circumference ≥ 94 cm in men and ≥ 80 cm in women as well as at least two of the following four components: (1) elevated triglycerides ( > 150 mg/dL) (2) reduced HDL cholesterol ( < 40 mg/dL in males and < 50 mg/dL for females), (3) increased blood pressure (BP) (systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg) and (4) raised fasting plasma glucose ( ≥ 100 mg/dL) (11).
3.5. Statistical Analysis
Statistical analysis of various parameters was performed using SPSS, version 22.0 (IBM Corporation, Armonk, New York, US). The associations between the clinical variables and CKD were estimated using both univariate and multiple logistic regression analyses. Odds ratios (OR) and 95% confidence intervals (95% CI) were also obtained. P-value less than 0.05 was considered statistically significant.
4. Results
Overall, 819 individuals with a mean age of 43.0 ± 14.0 years (age range: 18 - 88 years) were recruited in this study. Of these participants, 340 (41.5%) were male and 479 (58.5%) were female. Table 1 shows the clinical characteristics of the study population. In general, male participants presented with significantly higher anthropometric indices than their female counterparts (P < 0.001), and only BMI was significantly higher in the female participants (P < 0.001). A significant gender difference was found in the levels of hemodynamic parameters measured in this study. Systolic and diastolic blood pressure was found to be significantly higher among the male participants than the female subjects (P < 0.001). The mean triglyceride level was observed to be higher in the male population compared to the female ones (P = 0.09). Fasting blood glucose levels were found to be comparable between both sexes (P = 0.18).
| Parameter | Total (N:819) | Male (N:340) | Female (N:479) | P Value |
|---|---|---|---|---|
| Age (range: 18 - 88), y | 43.0 (14.0) | 43.37 (13.31) | 42.82 (14.53) | 0.57 |
| Body height, cm | 163.7 (10.5) | 172.52 (8.45) | 157.57 (6.94) | < 0.001 |
| Body weight, kg | 70.0 (12.8) | 74.76 (12.90) | 66.65 (11.63) | < 0.001 |
| Body mass index, kg/m2 | 26.1 (4.4) | 25.11 (3.88) | 26.89 (4.71) | < 0.001 |
| Waist circumference, cm | 89.26 (11.42) | 92.57 (10.54) | 86.90 (11.45) | < 0.001 |
| Systolic blood pressure, mmHg | 115.5 (14.3) | 118.30 (13.51) | 113.53 (14.60) | < 0.001 |
| Diastolic blood pressure, mmHg | 74.4 (8.8) | 76.80 (8.26) | 72.74 (8.93) | < 0.001 |
| Serum albumin, g/dL | 4.5 (1.6) | 4.70 (2.44) | 4.46 (0.41) | 0.007 |
| Total cholesterol, mg/dL | 184.54 (41.96) | 180.54 (38.70) | 187.32 (43.91) | 0.02 |
| LDL-cholesterol, mg/dL | 106.89 (34.08) | 105.39 (32.88) | 107.94 (34.88) | 0.30 |
| Triglyceride, mg/dL | 140.8 (76.5) | 146.18 (84.51) | 137.02 (70.31) | 0.09 |
| HDL-cholesterol, mg/dL | 49.9 (11.0) | 47.16 (9.42) | 51.98 (11.64) | < 0.001 |
| Fasting plasma glucose, mg/dL | 93.1 (28.5) | 91.60 (23.36) | 94.21 (31.61) | 0.18 |
| Serum creatinine, mg/dL | 1.07 (0.6) | 1.16 (0.23) | 0.93 (0.20) | < 0.001 |
| eGFR, mL/min/1.73 m2 | 79.44 (21.24) | 80.18 (20.19) | 78.91 (21.96) | 0.40 |
| Metabolic syndrome, No. (%) | 212 (25.9) | 64 (18.8) | 148 (30.9) | < 0.001 |
| Individual component, No. (%) | ||||
| Abdominal obesity | 521 (63.6) | 159 (46.8) | 362 (75.6) | < 0.001 |
| Low HDL cholesterol | 292 (36.7) | 66 (20.1) | 226 (48.5) | < 0.001 |
| High fasting glucose | 172 (21.9) | 69 (21.3) | 103 (22.3) | 0.79 |
| High blood pressure | 207 (25.6) | 96 (28.7) | 111 (23.5) | < 0.001 |
| Hypertriglyceridemia | 252 (31.7) | 109 (33.1) | 143 (30.7) | 0.48 |
Abbreviations: eGFR, estimated glomerular filtration rates; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Values are expressed as mean (SD) unless otherwise indicated.
b Metabolic syndrome was defined according to IDF guidelines.
The overall prevalence of CKD (eGFR < 60 mL/min/1.73 m2) was 16.6% (men: 14% and women: 19.4%). Also, the prevalence rates of kidney function according to eGFR were 29.2% in eGFR ≥ 90, 53.7% in eGFR = 60 - 89, 15.5% in eGFR 30 - 59 and 1.1% in eGFR ≤ 30. The prevalence of CKD increased with age in both men and women, particularly in those aged 60 years and over.
The prevalence of MetS was 25.9% (30.9% in women and 18.8% in men), which was significantly higher among women (P < 0.001). Among the individual components of MetS, abdominal obesity (63.6%) was the most common risk factor followed by low HDL-cholesterol (36.7%), high triglyceride level (31.7%), high blood pressure (25.6%), and high fasting blood sugar (21.9%). The prevalence of high abdominal obesity was significantly tilted towards women (75.6% vs. 46.8%; P < 0.001). Women also had significantly lower HDL levels compared to men (48.5% vs.20.1%; P < 0.001). Hypertension was significantly more in men than in women. The rest of the MetS components were similar between the two genders (Table 1). The most frequent cluster of MetS components included low abdominal obesity, low HDL-C and hypertriglyceridemia. Abdominal obesity and low HDL could be considered as the early risk factors for MetS (Table 2).
| MetS Components Number | Metabolic Syndrome |
|---|---|
| N = 1 | |
| WC | 94 (44.13) |
| HDL | 67 (31.45) |
| TG | 22 (10.32) |
| BP | 17 (7.98) |
| FPG | 13 (6.10) |
| N = 2 | |
| WC, HDL | 80 (33.47) |
| BP, TG | 58 (24.26) |
| WC, BP | 34 (14.22) |
| WC, TG | 22 (9.20) |
| Others | 45 (18.8) |
| N = 3 | |
| WC, HDL, TG | 37 (25.34) |
| WC, BP, TG | 30 (20.54) |
| WC, FPG, TG | 23 (15.75) |
| WC, BP, FPG | 19 (13.01) |
| Others | 37 (25.32) |
| N = 4 | |
| WC, BP, TG, FPG | 17 (29.31) |
| WC, BP, FPG, HDL | 15 (25.86) |
| WC, BP, HDL, TG | 15 (25.86) |
| WC, FPG, HDL, TG | 9 (15/51) |
| WC, FPG, HDL, TG | 2 (3.44) |
| N = 5 | |
| WC, BP, FPG, HDL, TG | 15 (100) |
Abbreviations: BP, high blood pressure; FPG, high fasting plasma glucose; HDL, increased high-density lipoprotein; WC, high waist circumference; TG, high total glycerides.
a Values are expressed as No. (%).
The prevalence of CKD was higher in subjects with MetS than those without it (23% vs. 10.8%; P < 0.001; Table 3). The prevalence of MetS grew with an increase in CKD stage (P < 0.001). On the other hand, 47.4% of the participants with CKD had MetS, 80.1% had abdominal obesity, 45.6% hypertriglyceridemia, 33.1% low HDL cholesterol, 42.5% hypertension and 31.3% high fasting blood glucose.
| Without MetS | With MetS | P Value | |
|---|---|---|---|
| Number of subjects (%) | 607 (71.4) | 212 (25.9) | |
| Age, y | 37.9 (13.3) | 48.0 (13.1) | < 0.0001 |
| Body height, cm | 166.3 (10.4) | 161.3 (10.2) | < 0.0001 |
| Body weight, kg | 65.1 (11.5) | 74.6 (12.3) | < 0.0001 |
| Body mass index, kg/m2 | 23.4 (3.3) | 28.6 (3.9) | < 0.0001 |
| Waist circumference, cm | 82.9 (10.4) | 95.1 (8.9) | < 0.0001 |
| Systolic blood pressure, mmHg | 11.0 (11.7) | 119.2 (14.9) | < 0.0001 |
| Diastolic blood pressure, mmHg | 72.0 (7.7) | 76.5 (8.9) | < 0.0001 |
| Serum albumin, g/dL | 4.7 (2.2) | 4.4 (0.4) | 0.04 |
| Total cholesterol, mg/dL | 180.7 (40.4) | 195.3 (44.7) | < 0.0001 |
| Triglyceride, mg/dL | 111.1 (46.2) | 168.1 (87.7) | < 0.0001 |
| HDL-cholesterol, mg/dL | 51.6 (11.4) | 48.4 (10.4) | < 0.0001 |
| Fasting plasma glucose, mg/dL | 85.9 (17.4) | 14.8 (7.5) | < 0.0001 |
| eGFR (mL/min/1.73 m2) | 82.2 (20.8) | 72 (20.5) | < 0.0001 |
| CKD, No. (%) | 41 (10.8) | 93 (23.0) | < 0.0001 |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rates; HDL, high-density lipoprotein.
a Values are expressed as mean(SD) unless otherwise indicated.
The presence of MetS was associated with CKD with an increased odds ratio (OR) for CKD with a GFR of < 60 mL/min/1.73 m2 (OR: 3.07; 95% confidence interval [CI]: 2.09 - 4.50; P < 0.001). The prevalence of CKD increased with the number of MetS risk factors; the prevalence of CKD was 8.5% in those with no metabolic risk factors, 9.9% with one, 15.8% with two and 30.6% with three or more of the components (P < 0.001). ORs (95% confidence interval [CI]) were 1.189 (0.554 - 2.555; P = 0.657), 2.025 (0.990 - 4.141; P = 0.053) and 4.769 (2.413 - 9.424, P < 0.001) when the number of MetS risk factors increased from 1 to ≥ 3, respectively (reference was zero MetS risk factors). Univariate and multivariate-adjusted odds ratios of CKD associated with MetS and its components are presented in Table 4. In the multivariate methods with respect to age and sex, the analysis revealed the association of abdominal obesity, low HDL-C, hypertriglyceridemia and hypertension with CKD. Individuals with hypertension and abdominal obesity had higher ORs of increased susceptibility to CKD (Table 4).
| Prevalence of CKD | Unadjusted, OR (95%CI) | P Value | Age-Gender- Adjusted, OR (95% CI) | P Value | Multivariable Adjusted, OR (95%CI) | P Value | ||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| High triglyceride levels | 189 (28.8) | 62 (45.6) | 2.075 (1.423 - 3.026) | < 0.0001 | 1.479 (0.978 - 2.236) | 0.063 | 1.608 (1.078 - 2.398) | 0.020 |
| Abdominal obesity | 395 (60.1) | 109 (80.1) | 2.687 (1.709 - 4.197) | < 0.0001 | 1.419 (0.841 - 2.394) | 0.190 | 2.091 (1.290 - 3.391) | 0.003 |
| Low HDL level | 246 (37.4) | 45 (33.1) | 1.210 (0.819 - 1.789) | 0.312 | 1.262 (0.811 - 1.964) | 0.302 | 1.240 (.825 - 1.865) | 0.300 |
| Hypertension | 140 (21.6) | 57 (42.5) | 2.691 (1.822 - 3.975) | < 0.0001 | 1.021 (0.636 - 1.641) | 0.930 | 2.061 (1.364 - 3.113) | 0.001 |
| High blood glucose level | 129 (19.9) | 42 (31.3) | 1.840 (1.218 - 2.781) | 0.006 | 1.107 (0.696 - 1.763) | 0.667 | 1.281 (.826 - 1.986) | 0.269 |
Abbreviations: CKD, chronic kidney disease; HDL, high-density lipoprotein.
a Values are expressed as No. (%) unless otherwise indicated.
5. Discussion
The study showed an age-dependent increase in the prevalence of CKD (16.6%). The prevalence of CKD increased progressively with the number of MetS components from 8.5% of the subjects with no MetS components to 30.6% in subjects with three or more of the components. Each trait of MetS was associated with a high OR of CKD, with the exception of low HDL and high blood glucose. OR was higher for individuals with hypertension and central obesity.
In the present study, the prevalence of MetS was 25.9% that is similar to the rates reported in a previous study in Iran (12). The prevalence of MetS was significantly higher in women than in men and it increased with age. Another former study also showed that the prevalence of MetS increased with age and it was higher in women than in men (13). The higher prevalence of MetS among older adults in this study may be described by functional limitations, increased sedentary lifestyle and reduced physical activity among older adults as ascribed in other reports (14).
The overall prevalence of MetS observed in our study was lower than that reported in the United States (33%) (15). This discrepancy might be in part due to the differences in methods, population characteristics, age ranges and criteria used to define MetS among various studies. The frequency of individual components of MetS differed between various populations and ethnic groups (16). It is, therefore, necessary to assess the components of MetS to determine the pathogenesis of MetS in different countries.
In our study, abdominal obesity (63.6%) was the most common risk factor followed by low HDL-C (36.7%), high triglyceride level (31.7%), hypertension (25.6%) and high fasting blood sugar (21.9%). The most common component of MetS in the USA was obesity (84%) followed by hypertension (76%), low HDL-C (75%), high triglycerides (74%) and high glucose (41%) levels. Abdominal obesity was observed most frequently in individuals with MetS in our study, which is similar to the findings of a research performed in the United States (17).
To the best of our knowledge, this is the first study assessing the associations of individual MetS components, the presence of MetS and the number of MetS components with CKD among the Iranian population in southern Iran. The findings illustrated significant relationships between MetS, the presence of individual MetS components and the number of MetS components and CKD, independent of age or gender. MetS was found to be independently associated with an increased risk of CKD in population-based cohorts and cross-sectional studies (18). The prevalence of MetS in CKD patients was 30.2%; this result is consistent with those of prior studies that showed a high prevalence of MetS and significant association between MetS and CKD in individuals with CKD (19). In a study conducted in Southeastern Asia, the prevalence of MetS in advanced CKD patients was found to be 37.5% (20). Meta-analysis of MetS prevalence in patients with CKD showed that MetS was a significant determinant of CKD (4). Previous studies have linked each of the components of the MetS with an increased risk of CKD. However, these studies have yielded inconsistent results as to the association between MetS-related traits and the risk of CKD (21).
In the multivariate methods, our study demonstrated that abdominal obesity, high triglyceride levels and hypertension were associated with CKD with respect to age and sex. However, there was no association between CKD and low HDL level and high blood glucose. Our findings are consistent with the results reported by studies in which central obesity was associated with CKD (22). Hypertension is a well-established leading cause for the progression of CKD (23). Our findings were in accordance with those of previous studies showing high TG levels to be associated with CKD (24).
In multivariate methods with respect to age and sex, the results of our study illustrated that all the traits of MetS, except for low HDL and high blood glucose levels, were associated with CKD. Our finding was consistent with those of Landecho demonstrating no significant association between low HDL and CKD (25). Although it has been illustrated that low level of HDL is a risk factor for decline in GFR, low HDL-C (under 30 mg/dL) was associated with increased risk of the incidence of eGFR under 60 mL/min/1.73 m2. Moreover, the association of low HDL-C with the presence of CKD and microalbuminuria has not been ascertained (26).
This study showed that high blood glucose was significantly associated with CKD in univariate analysis. High blood glucose is closely related to age because the prevalence of diabetes increases with advancing age (27). In multivariable analysis, we further adjusted age and sex and found that the significance did not persist, indicating that the association between blood glucose and CKD was dependent on age. We confirmed the prevalence of CKD among individuals with MetS among the general Iranian population with a new and more validated CKD-EPI equation used for the first time in the Iranian population and provided new and important information regarding the relationship between MetS traits and the risk of CKD.
These findings warrant greater attention to policies and interventions, such as lifestyle modifications, intended to reduce the prevalence of MetS and its adverse outcomes. In this study, MetS according to the International Diabetes Federation (IDF) definition is associated with an increased risk of CKD. The Iranian National Committee of Obesity (INCO) has proposed revised criteria for MetS with three abnormal findings among five with the same variables of IDF criteria and cutoff points, except waist with regional cutoff value of waist circumference > 95 cm for men and women (28). Despite the similarity of IDF and INCO criteria, further studies using INCO criteria are needed to better understand the association of MetS and its various components with CKD in the Iranian population.
Our study had some limitations that are worth mentioning. First, as this was a cross-sectional study, it does not indicate any causality. Further prospective studies are required to confirm the associations and to investigate the potential impact of prevention and individual treatment programs for each MetS trait on MetS occurrence and progression of CKD in the Iranian population. Second, the level of kidney function was measured by estimated creatinine-based equation instead of measuring GFR directly.
In conclusion, the findings of our population-based study showed a high prevalence of MetS in southern Iran. The trend of gender vulnerability was towards the female sub-population. Our study illustrated MetS and its individual components, except for low level of HDL and high blood glucose, as strong and independent risk factor for CKD. There was a graded relationship between the number of MetS components and the risk of CKD.
