1. Context
Cardiovascular disease imposes a large health burden in patients with diabetes (1, 2). Type 2 diabetes is associated with about a twofold increase in the risk of a wide range of cardiovascular diseases (3). Mortality risk after the first incidence of myocardial infarction is higher in patients with diabetes than in those without diabetes (4). The prevention of cardiovascular events, therefore, is a major concern in the treatment of patients with diabetes.
Recently, one study in a university-affiliated clinic in Iran investigated the effect of antidiabetic medications, including metformin, glibenclamide plus metformin, insulin alone, and insulin plus metformin, on pulse pressure and blood pressure, showing no significant difference between any of these anti-hyperglycemic drugs (5). Unfortunately, there are no nationally representative data on the mortality of diabetes patients undergoing treatment with different oral antidiabetic drugs. We studied this issue in a relatively large sample in our diabetes center, which revealed that treatment with glyburide was correlated with all-cause mortality and cardiovascular mortality (6).
SGLT2 (sodium-glucose cotransporter type 2) inhibitors are new glucose-lowering agents. As recently reported, they can reduce the risk of major adverse cardiovascular events (MACE) and improve renal outcomes.
Diabetes is also associated with an increased risk of adverse renal events so that diabetic kidney disease is the leading cause of the end-stage renal disease (7, 8). Moreover, renal events are likely to influence cardiovascular outcomes (9). The aim of this narrative review was to discuss the novel findings of the effects of SGLT2 inhibitors on cardiovascular and renal outcomes of type 2 diabetes.
2. Evidence Acquisition
The literature published in PubMed, Scopus, Web of Science, Google Scholar, and Cochrane library was reviewed up to January 2019. The keywords including SGLT2 inhibitor, type 2 diabetes, cardiovascular effect, and renal effect were used in different combinations. We examined RCTs, observational studies, review articles, and systematic reviews.
3. Results
3.1. Chemical Structure of SGLT2 Inhibitors
Phlorizin, a C glucoside analog, is the first SGLT inhibitor with effects on the activity of both SGLT1 and SGLT2. It was first isolated from the root bark of apple trees in 1835 (10, 11). Then, the new C-aryl glucoside-derived SGLT2 inhibitors with non-hydrolysable C - C bond were discovered; thus, gliflozins are a novel class of glucose-lowering agents (12-14).
Dapagliflozin was developed in 2008. The selectivity of dapagliflozin is about 1200 folds higher in humans for SGLT2 than for SGLT1 (15). Dapagliflozin was first approved in Europe in 2012, and the FDA approved it in 2014. Canagliflozin was approved by the FDA in 2013, with more than 400-fold higher inhibitory activity for SGLT2 than for SGLT1 (16).
The third agent in the gliflozin class is empagliflozin. European Medicines Agency (EMA) and the FDA approved empagliflozin in 2014. Empagliflozin has a higher selectivity for SGLT2 than for SGLT1 (2700 folds) (17). Another agent, ertugliflozin, was approved by EMA and FDA in 2017 (Table 1). In recent years, next-generation SGLT2 inhibitors, including ipragliflozin, tofogliflozin, and luseogliflozin, have been approved in Japan. Other agents that are in the late-phase of clinical trials include bexagliflozin and sotagliflozin (both as SGLT2/SGLT1 inhibitors) (18).
| Effects | SGLT2 Inhibitors |
|---|---|
| Drugs | Canagliflozin, dapagliflozin, empagliflozin, ertuglifozin |
| Target organ | The proximal tubule of the kidney |
| Mechanism of action | Forced glycosuria, Insulin-independent action, Reduction in glucose toxicity |
| Reduction in HbA1c | 0.7% to 1.0% |
| Change in weight | Reduction |
| Risk of hypoglycemia | Low |
| Blood pressure | Reduction |
| Use in renal impairment | Not effective in eGFR < 45 mL/min/1.73 m2a |
| Cardiovascular safety | Proven (EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI) |
| Side effects | Genital infection, ketoacidosis, foot amputationb, fracturec |
Characteristics of SGLT2 Inhibitors
3.2. Mechanism of Action
The mechanism of glucose-lowering effects of SGLT2 inhibitors is the inhibition of glucose reabsorption in the proximal tubule of the kidney that leads to increased glucose excretion. The action of these agents is independent of insulin secretion (20). The SGLT2 expression is upregulated in hyperglycemia and the threshold for urinary glucose excretion is also higher (21). SGLT2 inhibitors usually lead to the excretion of 60 - 100 grams of glucose per day, leading to reduced glucose toxicity and insulin resistance (22). The glucose-lowering effect of SGLT2 inhibitors is glomerular filtration rate (GFR)-dependent, with an average of 0.79% reduction in HbA1c in normal renal function, 0.3% - 0.4% in the estimated GFR (eGFR) range of 30 - 59 mL/min/1.73 m2, and no effect in the eGFR < 30 mL/min/1.73 m2 (23). In the literature, a 0.7 - 1% reduction in HbA1C and 3 - 4 kg reduction in body weight were reported by using SGLT2 inhibitors (24). These agents also can lower blood pressure by modest diuresis and natriuresis (25).
3.3. Cardiovascular Effects
3.3.1. Composite of Triple MACE
Here, we briefly review the most recent trials of cardiovascular outcomes of SGLT2 inhibitors. The EMPA-REG OUTCOME study included 7020 participants with type 2 diabetes and previous cardiovascular disease (CVD) in two groups to receive empagliflozin or placebo added to standard treatment. Empagliflozin was associated with about 14% relative risk reduction in three-point MACE including cardiovascular death, non-fatal MI, and non-fatal stroke (P = 0.04 for superiority) (26).
The CANVAS (Canagliflozin Cardiovascular Assessment Study) program included data of 10142 participants with type 2 diabetes and high risk for cardiovascular events from two trials (CANVAS and CANVAS-R: Canagliflozin Cardiovascular Assessment Study-Renal). The patients randomly received canagliflozin (100 - 300 mg) or placebo and the mean follow-up time was 3.6 years. The rate of triple MACE was significantly lower in the canagliflozin treatment group than in the placebo group (hazard ratio (HR): 0.86; 95% CI: 0.75 - 0.97; P = 0.02 for superiority) (27).
These findings were confirmed in two observational studies of the CVD-REAL Nordic study; the first one compared SGLT2 inhibitors (94% with dapagliflozin) with other antidiabetic drugs (HR: 0.78; 95% CI: 0.69 - 0.87; P < 0.001) (28) and the second one compared the newly treated patients with dapagliflozin versus newly treated patients with DPP-4 inhibitors (HR: 0.79; 95% CI: 0.67 - 0.94; P = 0.006) (29).
The result of a randomized controlled trial with dapagliflozin, the DECLARE-TIMI 58 study, has recently been published. The important point of this study, distinguishing it from other trials on SGLT2 inhibitors, was that the majority of the recruited patients had no previous ASCVD. In this trial, the researchers included 17160 patients including 10186 patients with no atherosclerotic cardiovascular disease. Patients received randomly either placebo or dapagliflozin. The results of composite primary endpoints showed noninferiority of dapagliflozin to placebo (P < 0.001). The rate of MACE was 8.8% in dapagliflozin and 9.4% in placebo groups (HR: 0.93; CI: 0.84 - 1.03; P = 0.17) (30).
A meta-analysis of three large trials, including EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58, was conducted by Zelniker et al. in 2018 revealing an 11% reduction in MACE by using SGLT2 inhibitors (HR: 0.89; 95% CI: 0.83 - 0.96, P = 0.0014), and this effect only was seen in patients with previous atherosclerotic cardiovascular disease (HR: 0.86; 95% CI: 0.80 - 0.93) (31).
Wu et al. in a meta-analysis of 57 published trials, including 33385 patients and six regulatory submissions (37525 patients) and data for seven different SGLT2 inhibitors, revealed a significant reduction in primary composite cardiovascular endpoints (MACE) in patients treated with SGLT2 inhibitors compared to placebo (relative risk: 0.84; 95% CI: 0.75 - 0.95; P = 0.006) (32).
A recently published meta-analysis by Zhang et al. in 2018 including five randomized controlled trials with 351476 participants showed a 20% reduction in MACE with SGLT2 inhibitors (HR: 0.80; 95% CI: 0.69 - 0.92; P = 0.002) (19).
A meta-analysis by Tang et al. in 2016, including 37 trials and 29859 patients with diabetes, compared canagliflozin, dapagliflozin, and empagliflozin with placebo and other glucose-lowering treatments. The study showed that only was empagliflozin associated with a significantly lower risk of MACE compared to placebo (odds ratio (OR): 0.81; 95% CI: 0.70 - 0.93) (33).
3.3.2. Myocardial Infarction (Fatal or Non-Fatal)
Data about myocardial infarction (MI) in patients treated with SGLT2 inhibitors are heterogeneous. In the EMPA-REG OUTCOME trial, there was no significant reduction in MI among patients with type 2 diabetes treated with empagliflozin 10 or 25 mg compared to placebo (HR: 0.87; 95% CI: 0.70 - 1.09; P = 0.23) (26). Moreover, in the CANVAS trial, MI had not a significant reduction (HR: 0.85; 95% CI: 0.69 - 1.05; P > 0.05) (27), as the same as in two sub-studies using the CVD-REAL Nordic database (28, 29).
The sub-analysis of the CVD-REAL study showed a lower risk of MI with the initiation of SGLT2 inhibitors (HR: 0.85; 95% CI: 0.72 - 1.00; P = 0.05). Moreover, the CVD-REAL2 study, a large multinational study of patients with type 2 diabetes from six countries with over 400000 patients, confirmed this finding (HR: 0.81; 95% CI: 0.74 - 0.88; P < 0.001) (34, 35).
The rate of MI in the DECLARE-TIMI study was 4.6% in the dapagliflozin group and 5.1% in the placebo group (HR: 0.89; 95% CI: 0.77 - 1.01) (30). In a meta-analysis of 81 trials in 2016, SGLT2 inhibitors were not associated with lower risk of MI compared to placebo (OR: 0.89; 95% CI: 0.74 - 1.09; P = 0.29) (36). Zhang et al. in a meta-analysis showed that SGLT2 inhibitors significantly reduced the risk of non-fatal MI (HR: 0.86; 95% CI: 0.76 - 0.98; P = 0.02) (19). Two meta-analysis by Savarese et al. and Monami et al. showed a significant reduction in MI (relative risk (RR): 0.803; 95% CI: 0.668 - 0.965; P = 0.02 and HR: 0.77; 95% CI: 0.63-0.94; P = 0.01, respectively) (37, 38). Wu et al. in a meta-analysis of 57 randomized controlled trials demonstrated no significant reduction in MI (RR: 0.88; 95% CI: 0.72-1.07; P = 0.18) (32).
3.3.3. Stroke
There are also heterogeneous findings about stroke in patients treated with this novel therapy. The EMPA-REG OUTCOME trial did not show a significant reduction in stroke in patients with type 2 diabetes treated with empagliflozin 10 or 25 mg compared to placebo (HR:1.18; 95% CI 0.89 - 1.56; P = 0.26) (26). Moreover, in CANAVAS trial, no reduction in stroke was observed (HR: 0.9; 95% CI: 0.71 - 1.15; P = non-significant) (27), similar to two studies of CVD-REAL Nordic (HR: 0.86, 95% CI: 0.72 - 1.04; P = 0.113 and HR: 0.79; 95% CI: 0.61 - 1.03; P = 0.086) (37, 38).
The DECLARE-TIMI study showed that ischemic stroke had no significant difference between the dapagliflozin group and the placebo group (HR: 1.01; 95% CI: 0.84 - 1.21) (30). The CVD-REAL study showed a lower risk of stroke with the initiation of SGLT2 inhibitors in patients with diabetes (HR: 0.83; 95% CI: 0.71 - 0.97; P = 0.05). Moreover, the CVD-REAL2 study, a large multinational study of patients with type 2 diabetes, confirmed this finding (HR: 0.68; 95% CI, 0.55 - 0.84; P < 0.001) (34, 35).
A meta-analysis by Wu et al. noted an adverse effect for non-fatal stroke (RR: 1.30; 95% CI: 1.00 - 1.68; P = 0.049) (32). However, some meta-analysis studies showed no difference in the risk of stroke between SGLT2 inhibitors and placebo (19, 33, 36-40).
3.3.4. Heart Failure
Heart failure is an important comorbidity in type 2 diabetes, especially in older patients (41). A significant reduction in hospitalization for heart failure was observed in patients with type 2 diabetes treated with empagliflozin in the EMPA-REG OUTCOME trial (HR: 0.65; 95% CI: 0.50 - 0.85; P = 0.002) (26). This effect was noticed rapidly in the first six months of the initiation of treatment (42); however, in CANVAS trial, there was no significant reduction in hospitalization for heart failure (27). A systemic review and meta-analysis with trial sequential analysis by Zhang et al. in 2018 demonstrated a significant reduction in hospitalization for heart failure in patients receiving SGLT2 inhibitors (HR: 0.62; 95% CI: 0.55 - 0.69; P < 0.001) (19).
In patients treated with dapagliflozin in the DECLARE trial, the reduction rate of hospitalization for heart failure was the only cardiovascular outcome that showed superiority (HR: 0.73; 95% CI: 0.61-0.88) (30).
Zelniker et al. conducted a meta-analysis of three large trials of SGLT2 inhibitors, including EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58, and showed a 23% reduction in the risk of cardiovascular death or hospitalization for heart failure (HR: 0.77; 95% CI: 0.71 - 0.84, P < 0.0001) and this benefit was the same in patients with and without cardiovascular disease and patients with and without previous heart failure (31).
Moreover, the meta-analyses of trials by Savarese et al. and Saad et al. showed a significant reduction in hospitalization due to heart failure (HR: 0.652; 95% CI: 0.517-0.823; P < 0.001 and HR: 0.67; 95% CI, 0.51 - 0.87; P = 0.003, respectively) (36, 37); however, some meta-analyses of studies with the exclusion of CANVAS program and EMPA-REG OUTCOME did not show a reduction in hospitalization for heart failure (19, 37, 39, 40).
The CVD-REAL study of 309056 diabetic patients newly treated with SGLT2 inhibitors or other antidiabetic drugs in the United States, Norway, Denmark, Sweden, and the United Kingdom showed the real-world effectiveness of the initiation of SGLT2 inhibitors in reduced hospitalization due to heart failure (HR: 0.61; 95% CI: 0.51 - 0.73; P < 0.001) (43). Moreover, the CVD-REAL2 study, a large multinational study of patients with type 2 diabetes, confirmed this finding (HR: 0.64; 95% CI: 0.50 - 0.82; P = 0.001) (35), in line with the two studies of CVD-REAL Nordic (HR: 0.7; 95% CI: 0.7261-0.81; P < 0.001 and HR: 0.62; 95% CI: 0.50-0.77; P < 0.001) (29, 32). In a meta-analysis of 37 trials in 2016, only was empagliflozin associated with the lower incidence of heart failure or hospitalization due to heart failure (OR: 0.65; 95% CI: 0.50 - 0.84) (33).
3.3.5. Cardiovascular Mortality
Cardiovascular mortality rate was lower in patients with type 2 diabetes treated with SGLT2 inhibitors in the EMPA-REG OUTCOME trial (38% relative risk reduction) (HR: 0.62; 95% CI: 0.49 - 0.77; P < 0.001) (26). A similar finding was obtained in the observational CVD-REAL Nordic study that compared SGLT2 inhibitors with other antidiabetic agents (HR: 0.53; 95% CI: 0.40 - 0.71; P < 0.001) (28). Some meta-analyses also showed a reduction in cardiovascular mortality in patients receiving SGLT2 inhibitors (32, 36-38).
The reduction in the cardiovascular mortality rate was not significant in CANVAS program comparing canagliflozin with placebo (HR: 0.87; 95% CI: 0.72 - 1.06), in the DECLARE-TIMI trial comparing dapagliflozin with placebo (HR: 0.98; 95% CI: 0.82 - 1.17), and in the observational CVD-REAL Nordic study comparing dapagliflozin 10 mg with DPP4 inhibitors (HR: 0.76; 95% CI: 0.53-1.08; P = 0.122) (27, 29, 30). In a meta-analysis by Zhang et al. in 2018, there was a significant reduction in cardiovascular mortality in patients treated with SGLT2 inhibitors (HR: 0.77; 95% CI, 0.60 - 0.98; P = 0.033) (19).
3.3.6. All-Cause Mortality
A significant reduction in all-cause mortality was shown in patients receiving SGLT2 inhibitors compared to placebo in the EMPA-REG OUTCOME trial (32% relative risk reduction) (26). However, there was no significant reduction in all-cause mortality in CANVAS trial in patient receiving canagliflozin versus placebo (HR: 0.87; 95% CI: 0.74 - 1.01; P = 0.033) (27). Conducted by Tang et al., a meta-analysis of 37 trials involving 29859 patients compared canagliflozin, empagliflozin, and dapagliflozin with placebo and other glucose-lowering agents and demonstrated that empagliflozin was the only treatment lowering the risk of all-cause mortality (OR: 0.67; 95% CI: 0.56-0.81), indicating a protective effect (33).
In the DECLARE-TIMI trial, death from any cause was not significantly different between the dapagliflozin and placebo groups (6.2% in dapagliflozin and 6.6% in placebo; HR: 0.93; 95% CI: 0.82-1.04) (30).
Some other meta-analyses showed that SGLT2 inhibitors significantly reduced the risk of all-cause mortality (19, 32, 36-38). Moreover, observational studies, including CVD-REAL, CVD-REAL2, and CVD-REAL Nordic, confirmed these findings (28, 29, 35, 43).
3.3.7. Mechanisms of Cardiovascular Effects
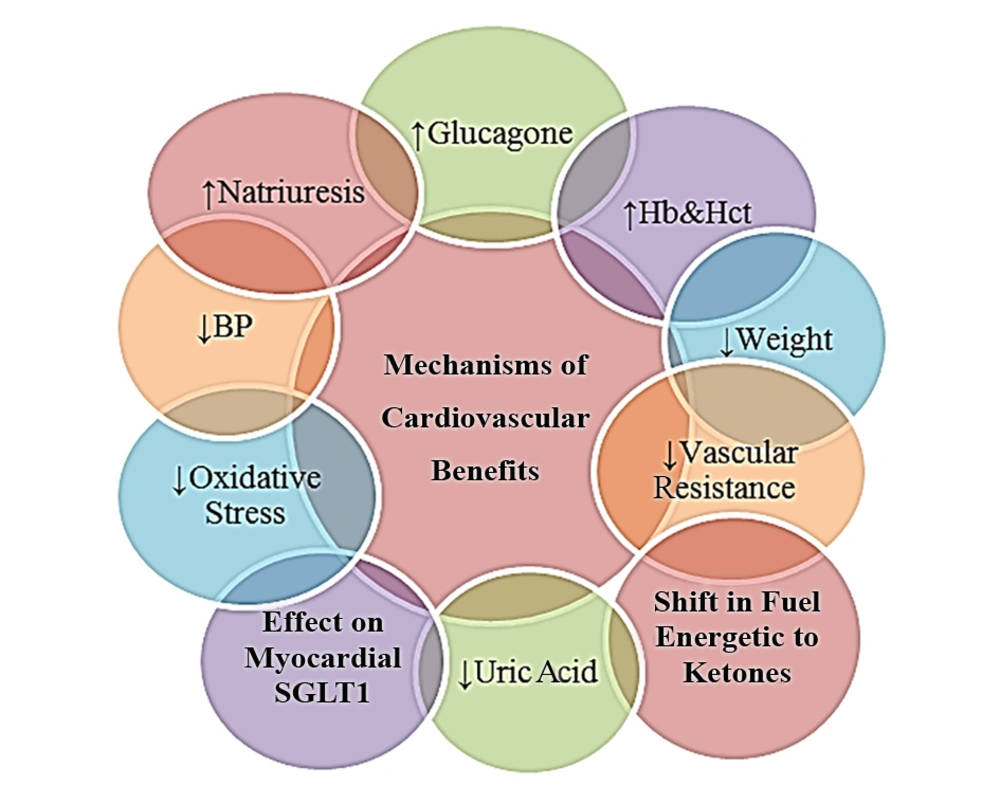
Several mechanisms have been proposed for cardiovascular effects of SGLT2 inhibitors, including osmotic diuresis and natriuresis leading to blood pressure lowering, decrease in arterial stiffness and vascular resistance, decrease in weight, and decrease in uric acid and oxidative stress. Increases in hemoglobin and hematocrit may also play a role. The EMPA REG trial showed a strong association between hemoglobin or hematocrit and reduced heart failure and the risk of death (26, 44-46). Glucagon regulates cardiac glucose utilization and has a positive inotropic and anti-arrhythmogenic effects (47). Empagliflozin can increase blood glucagon levels, possibly due to glucose excretion and/or exerting a direct effect on pancreatic alpha cells. The reduced risks of heart failure and cardiovascular mortality may be explained by the improvement of arrhythmia and myocardial function related to higher levels of glucagon (48-50). A recent hypothesis suggests that SGLT2 inhibitors may improve myocardial work efficiency by changing the fuel metabolism from free fatty acids to ketones, which are more efficient energetic fuels (51).
Another new theory suggests the reduction of cardiovascular mortality with empagliflozin may be due to a direct effect on cardiomyocytes to improve myocardial function and reduce rhythm disturbances. A weak inhibitory effect of empagliflozin on myocardial SGLT1, particularly in patients with overexpression of SGLT1 after ischemia, could contribute to the reduction of cardiovascular mortality (52). The cardiovascular benefits of SGLT2 inhibitors and probable mechanisms of these actions are summarized in Table 2 and Figure 1, respectively.
| Outcomes | EMPA - REG Trial | CANVAS Program | DECLARE - TIMI |
|---|---|---|---|
| Three-point MACE | 0.86 (0.74 - 0.99) | 0.86 (0.75 - 0.97) | 0.93 (0.84 - 1.03) |
| Myocardial infarction | 0.87 (0.70 - 1.09) | 0.89 (0.73 - 1.09) | 0.89 (0.77 - 1.01) |
| Stroke | 1.18 (0.89 - 1.56) | 0.87 (0.69 - 1.09) | 1.01 (0.84 - 1.21) |
| Hospitalization for heart failure | 0.65 (0.5 - 0.85) | 0.67 (0.52 - 0.87) | 0.73 (0.61 - 0.88) |
| Cardiovascular mortality | 0.62 (0.49 - 0.77) | 0.87 (0.72 - 1.06) | 0.98 (0.82 - 1.17) |
| All-cause mortality | 0.61 (0.53 - 0.70) | 0.87 (0.74 - 1.01) | 0.93 (0.82 - 1.04) |
Cardiovascular Outcomes in Three Major Trials of SGLT2 Inhibitorsa
3.4. Renal Effects of SGLT2 Inhibitors
3.4.1. Reduction in the Progression of Albuminuria
A significant relative risk reduction in progression to macroalbuminuria was observed in the empagliflozin group versus the placebo group in the EMPA-REG OUTCOME trial. It was shown that 11.2% of the patients in the empagliflozin group had progression to macroalbuminuria compared to 16.2% in the placebo group, with a 38% relative risk reduction (53). The CANVAS program showed a 27% reduction in the progression of albuminuria and also more regression of albuminuria compare to placebo (HR: 0.73; 95% CI: 0.67 - 0.79 and HR: 1.70; 95% CI: 1.51 - 1.91, respectively) (27).
A recent meta-analysis in 2018 by Seidu et al. including 40 randomized clinical trials revealed that the administration of SGLT2 inhibitors, particularly canagliflozin and empagliflozin, to patients with or without renal failure improved albuminuria and slowed the rate of progression to macroalbuminuria (54).
3.4.2. Reduction in Doubling of the Serum Creatinine
In the EMPA-REG OUTCOME trial, doubling of the serum creatinine level occurred in 1.5% of participants treated with empagliflozin compared to 2.3% in participants receiving placebo, with a 44% risk reduction (HR 0.56; 95% CI: 0.39 - 0.79; P < 0.001) (53).
A meta-analysis by Seidu et al. showed treatment with SGLT2 inhibitors was associated with initial increases in serum creatinine levels, followed by a return to baseline in patients with renal failure, but serum creatinine was preserved in those without renal failure (54).
3.4.3. The Lower Rate of Renal Replacement Therapy
In end-stage renal disease, patients require renal replacement therapy including dialysis or kidney transplant (55). In the EMPA-REG OUTCOME trial, the renal replacement therapy started in 0.3% of patients receiving empagliflozin compared to 0.6% in those receiving placebo, with a significant risk reduction of 55% in patients treated with empagliflozin (HR: 0.45; 95% CI: 0.21 - 0.97; P = 0.04) (53).
CANVAS program showed a 40% reduction in the composite renal outcome, including the requirement for kidney replacement therapy, eGFR, or mortality from renal causes (HR: 0.60; 95% CI: 0.47 - 0.77) (27).
3.4.4. Effects on Renal Function Over Time
In the EMPA-REG OUTCOME trial, an initial reduction occurred in the eGFR in the empagliflozin group. In the first four weeks, the eGFR decreased in the empagliflozin users, weekly decreases of 0.62 ± 0.04 mL/min/1.73 m2 in patients treated with empagliflozin 10 mg and 0.82 ± 0.04 mL/min/1.73 m2 in patients treated with empagliflozin 25 mg, compared to a small increase of GFR (0.01 ± 0.04 mL/min/1.73 m2) in patients receiving placebo. In a long-term follow-up, the eGFR remained stable in the empagliflozin group, but declined in patients receiving a placebo; the annual decreases of 0.19 ± 0.11 mL/min/1.73 m2 in empagliflozin users and a reduction of 1.67 ± 0.13 mL/min/1.73 m2 in placebo users were noted (P < 0.001) (53).
In the CANVAS program, after a decrease in the eGFR with canagliflozin (76 to 73 mL/min/1.73 m2) at three months, it remained stable for six years while the eGFR gradually declined with placebo (19).
In populations with renal impairment, SGLT2 inhibition was associated with an initial decline in the eGFR, followed by a return to baseline. In patients without renal impairment, an analysis of 17 studies showed no significant change in the eGFR with SGLT2 inhibitors compared to placebo (mean difference = 0.51 mL/min/1.73 m2; P = 0.403) (54).
In the DECLARE-TIMI trial, the composite renal outcome, including a ≥ 40% decrease in eGFR to lower than 60 mL/min/1.73 m2, decrease in end-stage renal disease, or decrease in death from renal causes, was improved in the dapagliflozin group than in the placebo group (HR: 0.53; 95% CI: 0.43 - 0.66) (30).
The meta-analysis of three trials, including EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58, revealed a 45% reduction in the risk of progression of renal disease (HR: 0.55; 95% CI: 0.48 - 0.64, P < 0.0001) and this benefit was similar in patients with and without atherosclerotic cardiovascular disease (31).
3.4.5. Mechanisms of Renal Effects of SGLT2 Inhibitors
The renal protection mechanisms are multifactorial. SGLT2 inhibitors reduce the reabsorption of sodium in the proximal tubule of the kidney and increase the delivery of sodium to the macula densa; this may restore the tubular glomerular feedback, leading to a reduction in the kidney blood flow, a decrease in glomerular hyperfiltration, and a reduction in intra-glomerular pressure (44). These effects may result in an acute decrease in albuminuria and eGFR, followed by eGFR stability in the long run (56).
As previously discussed, SGLT2 inhibitors have beneficial effects on multiple risk factors of renal impairment, including high blood glucose, high blood pressure, high serum uric acid, and body weight (23). These suggest the potential nephroprotective effect of SGLT2 inhibitors in patients with diabetes. The renal effects of SGLT2 inhibitors are summarized in Table 3.
| Renal Outcome | EMPA REG Trial Related Risk Reduction, % | CANVAS Program Related Risk Reduction, % | DECLARE-TIMI Risk Reduction, % |
|---|---|---|---|
| Progression of albuminuria | 38 | 27 | NA |
| Doubling of serum creatinine | 44 | NA | NA |
| The rate of renal replacement therapy | 55 | 40a | NA |
| Effects on renal function | A short-term decrease in eGFR in the first four weeks, followed by eGFR stability in the long-term follow-up | A decrease in eGFR at three months, followed by stability in a six-year follow-up | NA |
| Composite renal outcomea | NA | 40 | 47b |
Renal Outcomes in Three Major Trials of SGLT2 Inhibitors
4. Conclusions
Given the suboptimal glycemic and the importance of cardiovascular and renal risk reduction in type 2 diabetes and the high burden of cardiovascular and renal disease in patients with diabetes, novel therapies such as SGLT2 inhibitors seem to have an important clinical advantage to improve glycemic control and cardiovascular and renal outcomes.