1. Background
Graves’ ophthalmopathy (GO) is a major and the most frequent extrathyroidal manifestation of Graves’ syndrome. GO is an autoimmune disease related to the production of cytokines by T lymphocytes activated against TSH receptor, expressed by extraorbital tissues. Principally, the edema and swelling of the extraorbital tissue are determined by glycosaminoglycan and hydrophilic molecules produced by the stimulated fibroblasts and T cell infiltration. Typical symptoms at presentation include any combination of proptosis, pain, tearing, orbital movement impairment, with or without diplopia, periorbital edema, visual impairment, and rarely blindness (1).
Treatment options depend on the severity of the disease. In mild ophthalmopathy, local measures such as lubricants, sunglasses, and prisms are sufficient to spontaneous recovery. In a threatening ophthalmopathy, the treatment depends on either prednisone pulse therapy or on immediate surgical orbital decompression (2). The purpose of the treatment in patients affected by GO is to reduce the local immune response that can be achieved by controlling thyroid disease, with the administration of steroids or immunosuppressive therapy (3, 4). Sometimes systemic therapy with steroids can be ineffective and relapses of the disease are frequently observed. Under these circumstances, additional treatments such as surgery or radiotherapy (RT) are commonly needed (5, 6).
Over a century, RT has been used in the treatment of GO (7, 8). The rationale of using RT is based on the sensitivity to small doses of radiation of T lymphocytes and fibroblasts, which are mostly responsible for edema and fibrosis, respectively. Symptoms improvement or stabilization rates of around 94% - 97% can be achieved in GO after radiation treatment, especially when combined to steroids (9, 10). However, subjective and objective symptoms showed different responses to RT. Orbital movements, orbital pain, and tearing present the most robust response ranging from 25.5% to 65.8%. In contrast, vision loss and proptosis are the most refractory symptoms to RT with improvement rates ranging from 37.1% to 41.5% (11, 12). The overall salvage surgery rate after RT is about 50% (13, 14).
2. Objectives
The aim of this study is to evaluate the long term outcome of orbital RT with concomitant systemic steroids in a series of patients affected by moderate-to-severe GO not responsive/resistant to high dose systemic steroids or with severe diplopia.
3. Methods
3.1. Patients’ Characteristics
Between January 2009 and December 2016, 40 patients with moderate-to-severe GO not responsive/resistant to high dose steroids or with severe diplopia were recruited to the Department of Radiation Oncology. Disease activity was measured using the seven-items clinical activity score (CAS) (15) that consists of seven clinical items (spontaneous orbital pain, pain with eye movement, eyelid erythema, eyelid edema, conjunctival injection, chemosis, and swelling of the caruncle); 1 point was given to each of these clinical items and the total score might range from 0 (no activity) to 7 (very high activity). The disease was defined moderate-to-severe when presenting with CAS ≥ 4.
Inclusion criteria for radiotherapy were: CAS ≥ 4; evidence of severe diplopia regardless of CAS; disease not responsive/resistant to high-dose steroids. All patients included in this study were evaluated in a multidisciplinary team consisting of endocrinologists, ophthalmologists, maxillofacial surgeons, radiation oncologists, and neuroradiologists before any therapeutic decision.
Workup was completed with patients’ history and physical examination, thyroid disease history, prior corticosteroid use, ophthalmologist evaluation, surgery, and thyroid laboratory tests. Orbit-scan MRI (Magnetic resonance imaging) was performed for each patient to better define the severity of the disease (optical nerve compression) and to exclude other developing causes of the ophthalmopathy such as infection or tumor. Written informed consent was obtained from the patients.
3.2. Treatment
Radiation therapy was planned using a 3D conformal technique delivered through a Varian LINAC (6 MV photons) with 2 opposed beams. The clinical target volume (CTV) encompassed the bilateral orbits from the apex of the sphenoid sinus posteriorly to the fleshy cantus anteriorly and from the roof of the orbit superiorly to its floor inferiorly. A margin of 5 - 8 mm in all directions was added to the CTV to generate the planning target volume (PTV). The dose-volume histogram was in accordance with the accepted tolerance dose for organs at risk. The total prescribed dose to the PTV was 20Gy delivered in 10 fractions of 2Gy daily for five days a week over 2 weeks (6). All patients received concurrent high dose steroids that consisted of methylprednisolone i.v. 500 mg weekly.
3.3. Follow Up
The severity and activity of the disease were scored before the administration of the combined treatment by the ophthalmologist’s evaluation according to the EUGOGO consensus and the seven-item- CAS (16). Symptoms were classified as present (score 1) or absent (score 0), based on the ophthalmologist’s evaluation of the disease. Regular follow-up was performed by endocrinologist, ophthalmologist, and radiation oncologist for all patients.
All patients underwent weekly clinical evaluation during radiation therapy to register RT-related adverse effects according to Radiation Therapy Oncology Group (RTOG) scale (17).
3.4. Definitions
Treatment response was evaluated by an ophthalmologist at 6 and 12 months and each year afterward. Response to treatment was defined according to Sisti et al. (9). Responders were defined GO when at least one of the following criteria was fulfilled: (1) Reduction in proptosis ≥ 2 mm in at least one eye, with no increase ≥ 2 mm in the other eye; (2) reduction of at least CAS 2/7points; (3) reduction in eyelid aperture ≥ 2 mm in at least one eye, with no increase ≥ 2 mm in the other eye; (4) disappearance or improvement of diplopia (change of degree from constant to inconstant or intermittent, or from inconstant to intermittent); (5) increase in visual acuity ≥ 2/10. Non-responders were defined GO when at least one of the following criteria was fulfilled: (1) Increase of CAS ≥ 1/7 points; (2) increase in proptosis ≥ 2 mm; (3) increase in eyelid aperture ≥ 2 mm; (4) appearance or worsening (change of degree) of diplopia; (5) decrease in visual acuity ≥ 2/10.
Both eyes were often not affected by the same grade of GO. Data for the worse eye were reported and used for analysis.
3.5. Statistical Analysis
A univariate model using a χ2 test (in 2x2 contingency tables) was performed to correlate the resolution of each symptom and CAS at baseline and after 1 year and to correlate baseline characteristics, including sex, age ≥ 50 years, status of hyperthyroidism pre and post RT and surgical intervention pre and post RT; with the severity symptoms and CAS at baseline. A non-parametric Wilcoxon’s rank test was performed to correlate baseline characteristics and the severity of symptoms and CAS at presentation, and the resolution of each symptom and CAS at baseline and after 1 year. The statistical analysis was performed using SPSS software version 22.0 (IBM, Chicago, IL). A P value ≤ 0.05 was considered statistically significant.
4. Results
4.1. Patients’ Characteristics
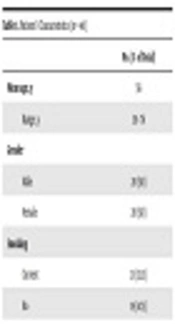
Patients’ characteristics are summarized in Table 1. Twenty (50%) patients were female, 20 (50%) were male. Mean age was 56 (range 39 - 76) and 21 (51.2%) patients were active smokers. The median time-duration of the GO before RT was 21 months (range 6 - 30 months). Median TSH before RT was 2.51 mU/L (0.02 - 69.5 mU/L), median fT3 was 3.12 pm/L (1.81-6 pm/L) and median fT4 was 0.87 ng/dl (0.5 - 1.96 ng/dl). Orbital decompression before RT was performed in one patient for severe optical nerve compression associated with acute blindness.
| Values | |
|---|---|
| Mean age, y (range) | 56 (39 - 76) |
| Gender | |
| Male | 20 (50) |
| Female | 20 (50) |
| Smoking | |
| Current | 21 (52.5) |
| No | 19 (47.5) |
| Diabetes | 4 (10) |
| Hypertension | 18 (45) |
| Active hyperthyroidism pre-RT | |
| Yes | 16 (40) |
| No | 24 (60) |
| Pre-RT TSHb | 2.51 mU/L (0.02 - 69.5) |
| Pre-RT fT3b | 3.12 pm/L (1.81 - 6) |
| Pre-RT fT4b | 0.87 ng/dL (0.5 - 1.96) |
| Surgical thyroid resection | 21 (52.5) |
| Duration of ophthalmopathy, months (range) | 21 (6 - 30) |
| Mean CAS pre-RT (range) | 5 (1 - 7) |
| CAS 1 - 3 | 6 (15) |
| CAS 4,5 | 27 (67.5) |
| CAS 6,7 | 7 (17.5) |
Abbreviations: CAS, clinical activity score; RT, radiotherapy.
a All data are presented as No. (%) unless otherwise specified.
b Median (interquartile range).
Median CAS at diagnosis was 5 (range 1 - 7). The most common symptoms at presentation were eyelid edema (90%), conjunctival injection (90%), lachrymation (85%), and eye movement impairment (82.5%). Clinical diplopia was present in 32 (80%) patients (Table 2).
| Features with Ophthalmopathya | Baseline | 3 - 6 Months After RT | 1 Year After RT | Resolution of Symptom at 1 Year (%) | P |
|---|---|---|---|---|---|
| CAS, median | 5 | 4 | 3 | - | < 0.001 |
| Proptosis, mm (range) | 21 (16 - 26) | 20.75 (17 - 25) | 20.75 (16 - 25) | - | n.s. |
| Active disease (CAS≥4) | 34 (85) | 23 (57.5) | 17 (42.5) | 42.5 | < 0.001 |
| Conjunctival injection | 36 (90) | 30 (75) | 33 (82.5) | 7.5 | n.s. |
| Eyelid edema | 36 (90) | 32 (80) | 23 (57.5) | 32.5 | < 0.001 |
| Lacrimation | 34 (85) | 28 (70) | 21 (52.5) | 32.5 | < 0.001 |
| Eye movements impairment | 33 (82.5) | 26 (65) | 16 (40) | 42.5 | < 0.001 |
| None | 8 (20) | 14 (35) | 21 (52.5) | 32.5 | - |
| Incostant | 18 (45) | 15 (37.5) | 13 (32.5) | - 12.5 | - |
| Costant | 14 (35) | 11 (27.5) | 6 (15) | - 20 | - |
| Photophobia | 33 (82.5) | 31 (77.5) | 30 (75) | 7.5 | n.s |
| Diplopia | 32 (80) | 26 (65) | 19 (47.5) | 32.5 | < 0.001 |
| Eyelid hyperemia | 32 (80) | 26 (65) | 21 (52.5) | 27.5 | < 0.001 |
| Itching | 30 (75) | 26 (65) | 23 (57.5) | 17.5 | n.s. |
| Chemosis | 29 (72.5) | 30 (75) | 19 (47.5) | 25 | 0.02 |
| Plica edema | 28 (70) | 21 (52.5) | 18 (45) | 25 | 0.02 |
| Grit sensation | 27 (67.5) | 20 (50) | 21 (52.5) | 15 | n.s. |
| Orbital pain | 13 (32.5) | 13 (32.5) | 7 (17.5) | 15 | n.s. |
| Orbital pain during movements | 14 (35) | 13 (32.5) | 9 (22.5) | 12.5 | n.s. |
| Orbital pain (grade≥2) | 7 (17.5) | 4 (10) | 5 (12.5) | 5 | n.s. |
| Orbital pain during movements (grade≥2) | 10 (25) | 8 (20) | 5 (12.5) | 12.5 | n.s. |
| Median visual acuity (range) | 10/10 (1 - 12/10) | 11/10 (1 - 11/10) | 11/10 (1 - 11/10) | - | n.s. |
| Improvement | - | - | 22 (27.5) | - | - |
| No changes | - | - | 32 (40) | - | - |
| Worsening | - | - | 26 (32.5) | - | - |
Abbreviations: CAS, clinical activity score; n.s., not significant.
a Of any grade if not specified.
b All data are presented as No. (%) unless otherwise specified.
4.2. Early Response to Treatment and Symptoms Control
Median follow-up after RT completion was 56 months (range 12 - 96 months). At 1 year, 28 patients (70%) experienced a reduction of CAS and 23 (57.5%) patients had inactive disease (CAS ≤ 3); 8 (20%) patients had stable disease and 4 (10%) presented with worsening symptoms. Overall, at 1-year CAS improved of a median of 2 points (5 vs. 3; P = < 0.001). Ten (25%) patients experienced an initial worsening of the GO at 6 months but at 1 year, 7 of them had an improvement and 3 patients had stable disease.
Overall, it was reported an improvement of symptoms at 1 year, in particular, eye movements in 42.5% of cases (P = < 0.001), diplopia 32.5% (P = < 0.001), lachrymation 32.5% (P = < 0.001), and eyelid edema 32.5 (P = < 0.001). Response to treatment and symptoms control are shown in Table 2.
Proptosis was stable before and after RT (21 mm, range 16 - 26, vs. 20.75 mm, range 16 - 25). Median visual acuity before RT (80 treated eyes in 40 patients) was 10/10 (range 1 - 12/10), while at 1 year was 11/10 (range 1 - 11/10). Improvement of eyesight at 1 year was reported in 22 (27.5%) eyes (range 2 - 10/10 vs. 7 - 11/10) and no changes in 32 (40%) eyes. A worsening in eyesight was reported in 26 (32.5%) eyes (range 7 - 12/10 vs. 4 - 11/10) of which: 14 (17.5%) eyesight loss 1/10 at 1 year and 12 (15%) eyesight loss 2 - 4/10 at 1 year. Eyesight loss at 1 year was not statistically significant.
4.3. Long Term Results
After a median 56-month-follow-up, GO was controlled in 29 (72.5%) patients. Seventeen (42.5%) patients received surgical intervention: Eleven (27.5%) of these patients experienced a relapse of disease and were treated with systemic steroids and ten (25%) of these patients showed no response after medical treatment and underwent orbital decompression (3 patients in the first 2 years of follow-up and 7 patients before the fifth year). Four (10%) patients received the surgical correction of the strabismus, 4 (10%) underwent surgery for bilateral cataract, and 1 (2.5%) patient underwent an eyelid lipectomy for the persistence of eyelid edema.
At the univariate analysis CAS score pre- and post-RT, response to treatment, sex, smoking status, and thyroid disease status did not predict the risk of pursuit a surgical intervention. No correlations was observed between response to treatment and sex, age ≥ 50 years, smoking, diabetes, hypertension, duration of GO, status of thyroid disease pre- and post-RT, surgical intervention pre- and post-RT, the severity of symptoms, and the time needed to respond to the treatment.
4.4. Toxicity
RT associated with systemic steroids was well tolerated. Grade 1 - 2 keratitis occurred in 8 (19.5%) patients. One patient experienced Grade 3 corneal ulcer 1 month after RT completion and received medical therapy with no sequelae. Late toxicity included Grade 1 - 2 xerophthalmia in 10 (25%) patients and 4 (10%) cases of monolateral cataract at 2 years. No secondary malignancies were reported.
5. Discussion
The natural history of GO is characterized by two phases: An active phase in which inflammation, lymphocyte and fibroblast infiltration are predominant and an inactive fibrotic phase. The clinical manifestation of untreated disease is described by the Rundles’ curve (18). During the active phase, RT plays a role after the failure of medical treatments (steroids and immunosuppressive therapy), especially in association with steroids (19-24). We reported the good outcome, acute and late side effects of orbital radiotherapy plus concomitant steroids in a series of patients affected by moderate-to-severe GO after a long-term follow-up.
Kim et al. (25) reported that orbital RT and concomitant steroids had a superior efficacy in reducing GO severity than steroids alone when evaluated by NOSPECS classification. The concomitant group showed 11.8% (8/68 patients) relapse within 1 year after treatment and the steroids alone group showed 28.8% (17/59 patients) relapse. In particular, ocular motility impairment was significantly improved by concomitant treatment in comparison to steroids alone group. Bartalena (26) randomized 48 patients with moderate-to-severe disease to receive RT plus steroids or steroids alone. After 1 year a very good response was described in the combined group; in particular, a soft tissue involvement was improved. Moreover, a meta-analysis reported the superiority of the concomitant regimen compared to RT alone (OR 17.5, 95% CI: 1.22 - 250; P = 0.04) in patients with moderate-to-severe GO (27).
In the current study, at 1 year an improvement was observed in eye movements of 42.5% (82.5% vs. 40%), diplopia of 32.5% (80% vs. 47.5%), and eyelid edema of 32.5% (90% vs. 57.5%) of the patients. Lacrimation recovered in 32.5% of the patients (85% vs. 52.5%) but this result could be partially related to the radiation damage to lachrymal glands. These results, despite the paucity of a control group, are comparable with recent findings in a similar population of the patients (10). Tanda and Bartalena also reported that RT is effective in the reduction of ocular motility impairment and diplopia but not proptosis or eyelid swelling (8).
Seven-item-CAS has been showed to be an easy and helpful tool for evaluating disease activity and response to immunosuppressive therapy; however, it is not accurate in describing the overall status of GO (28). For this reason, it is important to take into account the modifications of each symptom, as well.
In the current study, after 1 year a median of 2 points CAS reduction (5 vs. 3) and a translation to inactive disease in 57.5% of cases has been found. Also, CAS reduction was in accordance with symptom recovery. The population of our study was homogeneous, all patients had moderate-to-severe active phase GO or severe diplopia regardless of the CAS and all received steroids before and during RT. Radiation treatment was administered when steroids were ineffective or patients presented severe diplopia and low quality of life due to the symptoms. After a median follow-up of 56 months, the reactivation of the disease was observed in 11 (32.5%) patients. Salvage medical treatment was administered but 10 patients relapsed again and were then submitted to orbital decompression. These results are in accordance with other recent reports with long-term follow-up (9, 28, 29). Conversely, the results of the CIRTED study, a recent multicenter, 2 × 2 factorial, double-blind, randomized controlled trial reported no advantage of radiotherapy over sham radiotherapy and azathioprine. Moreover, azathioprine showed a benefit in a post-hoc analysis due to the high rate of withdrawals (30). The results of this study cannot be directly compared with ours because all patients in our study received treatment with steroids prior to orbital RT and irradiation was performed only when steroids were ineffective or in case of severe symptoms while only 12.6% patients of the previous study received steroids prior to orbital RT.
The absolute usage of salvage surgery after combined treatment in our studied population was 25% but no factors were found to be predictive for surgical intervention. Interestingly, the majority of salvage surgeries (7/10 cases) were performed in the late phase of FUP (3 - 5 years), reflecting, the chronic behavior of the disease with alternation of active phases and inactive phases, also with a long period of free-from-disease time (was not statistically confirmed). The possibility to identify the predictive factors of relapse could help the selected patients to undergo a more intensive or longer FUP.
Recently, Sisti et al. (9, 18) analyzed the results of a series of patients with mild-to-severe GO who underwent orbital RT plus steroids. After a 55.5-month-follow-up, the total rate of responders was 67.7%. Female gender was significantly associated with a higher prevalence of response (76.4% vs. 48%; P = 0.02). In our study, no factors were found to be predictive of response or relapse, also in the long term follow-up.
Acute toxicity was mild. Bilateral cataract occurred in 3 (7.4%) patients and grade 1 - 2 chronic dry eyes in 5 (12.5%) patients. These results are in line with previous studies (29, 31). No secondary malignancies occurred within the treatment field.
Nevertheless, the current study presents a number of limitations: In addition to the retrospective nature of the study and the absence of a control group, data regarding serum anti-TSH receptor autoantibodies and dose/duration of pre-RT high-dose steroids were not available. Points of strength are the homogeneity of the population and treatment, as well as the long-term follow-up. Our results confirm the good outcome and tolerability of the combination of orbital RT and systemic steroids administered in a homogeneous population of unfavorable patients. This regimen of treatment was found to be effective even after long-term follow-up; however, no predictive factors related to the relapse have clearly been found yet. Further studies in larger series are needed to evaluate the real efficacy of this schema and determine predictive factors in order to select subgroups that could benefit considerably from such therapy.
5.1. Conclusions
Our results confirmed the efficacy of the orbital RT combined with systemic corticosteroids in patients with moderate-to-severe GO or with severe diplopia, previously treated with high-dose steroids. Potential predictive factors could help clinicians to better select candidates, who could more benefit from this regimen. Long term results need to be validated in larger series.