1. Context
Chronic kidney disease (CKD), is correlated with a substantial upsurge in mortality and morbidity worldwide. Importantly, there has been a rise in its prevalence and incidence all over the world (1). The kidney disease outcome quality initiative (K/DOQI) guideline, defines chronic kidney disease as either kidney damage or glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 (1.0 mL/s/ 1.73 m2) for > 3 months (2).
A number of risk factors are known to affect CKD. They include female gender, age, anthropometric indices, smoking, diabetes mellitus, hypertension, and dyslipidemia. On the other hand, chronic kidney disease is associated with a number of metabolic and cardiovascular disorders. Also, electrocardiographic (ECG) changes are common in patients with CKD; however, there are still uncertainties about whether or not ECG disturbances can predict future cardiovascular events in these patients (3).
The aim of this study was to review the 20 years results of the Tehran lipid and glucose study (TLGS) on CKD and its association with metabolic and cardiovascular diseases.
2. Evidence Acquisition
We conducted a systematic review of all TLGS-based studies addressing chronic kidney disease. The terms “chronic kidney disease” AND “Tehran lipid and glucose study” were used to search in PubMed/Medline. All articles with the term “chronic kidney disease” in their title, subject or MeSh were included for the initial review. Since studies on nutrition issues as their main topic have been addressed elsewhere, we excluded all articles that had examined the relationship between CKD and nutrition from this review.
2.1. Definitions and Classification of CKD
GFR is estimated by a number of equations. One of the most commonly used ones is the abbreviated modification of diet in renal disease (MDRD) equation. The equation is expressed as:
GFR = 186 × (SCr)-1.154 × (Age)-0.203 × (0.742 if female) × (1.210 if African-American)
In this equation, GFR is expressed as mL/min per 1.73 m2, and serum creatinine (Scr) is expressed as mg/dL (1). Although the MDRD equation is quite popular, the CKD epidemiology collaboration (CKD-EPI) equation estimate GFR more accurately in individuals with either normal, mildly reduced, or even elevated GFR. Moreover, the CKD-EPI equation has resulted in diagnosing fewer individuals with CKD and more precise risk estimation for mortality than the MDRD equation in extremely large and broad populations. Previous studies compared the values of directly measured and eGFR. Their results showed that the CKD-EPI equation to estimate the GFR has been the most recommended creatinine-based alternative method for directly measured GFR to categorize CKD and assessment of the related risk factors (4). Using the CKD-EPI equation GFR is estimated as:
eGFR (mL/min per 1.73 m2) = 141 × minimum (Scr/κ, 1) α × maximum (Scr/κ, 1) - 1.209 × 0.993 Age × 1.018 [if female], where κ = 0.7 for females and 0.9 for males, α = -0.329 for females and -0.411 for males (5). The classification of CKD by stages is precisely defined by criteria of the kidney disease outcome quality initiative (K/DOQI) (1).
3. Results
3.1. Prevalence
Using the abbreviated MDRD study equation, Hosseinpanah et al. found that prevalence of CKD was 18.9% (95% confidence interval (CI): 18.2, 20.6). Accordingly, they found age adjusted CKD prevalence to be 14.9% (CI: 14.2, 15.6). Risk factors correlated with CKD comprised of age (odds ratio (OR) = 1.1, CI: 1.0, 1.2), gender (reference, male) (OR = 3.1, CI: 2.6, 3.7), Body mass index (BMI) (OR = 1.5, CI: 1.3, 1.8 for BMI 25 to < 30, and OR = 1.6, CI: 1.3, 2.0 for BMI ≥ 30), increased waist circumference (OR = 1.2, CI: 1.1, 1.4), hypertension (OR = 1.2, CI: 1.1, 1.4), and dyslipidemia (OR = 1.3, CI: 1.1, 1.5) (1).
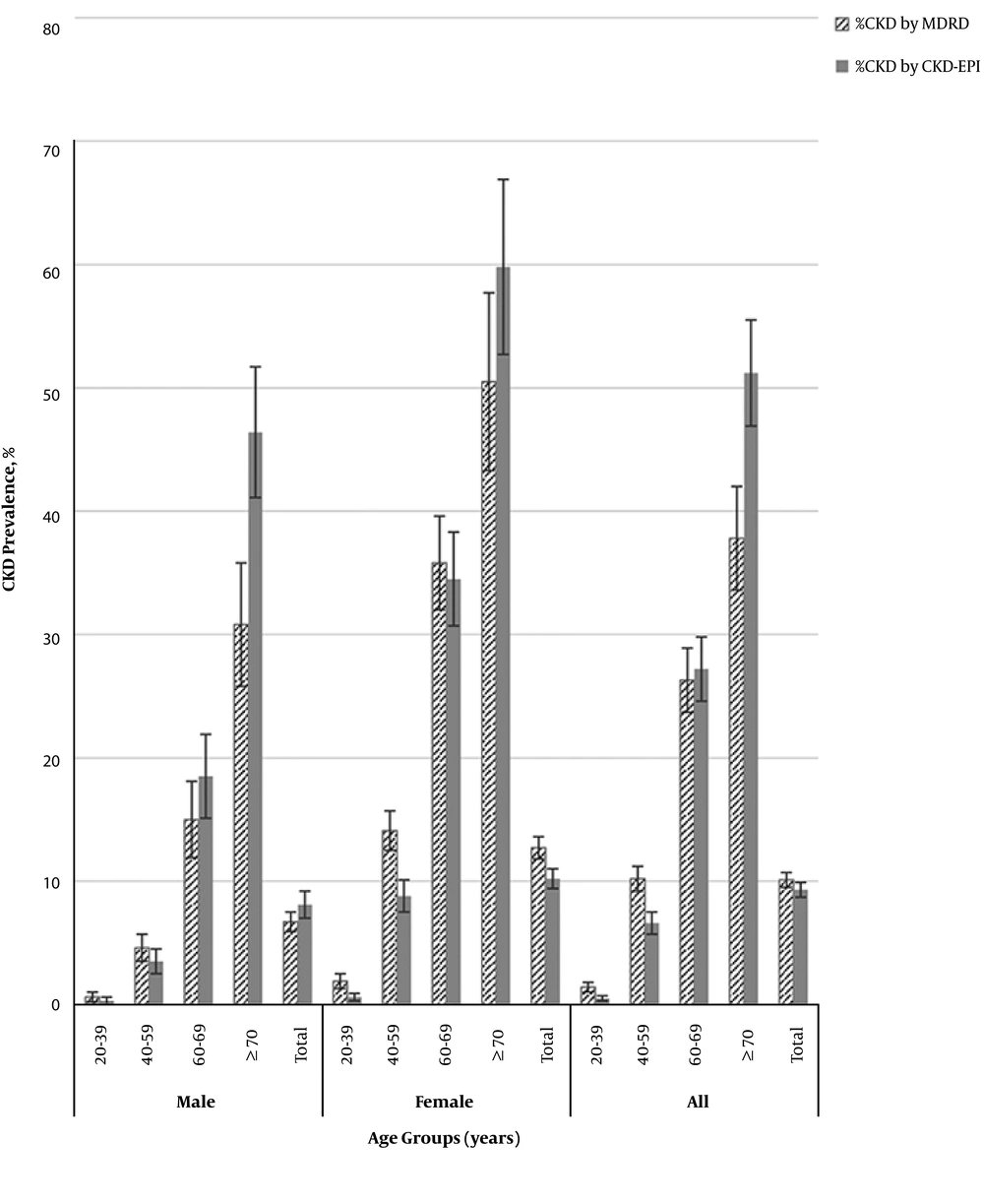
Another unpublished study aimed to estimate the prevalence of CKD through a cross-sectional study in a population selected from the Tehran lipid and glucose study (phase II) by calculating GFR using CKD-EPI and MDRD equations separately. Data of a population of 8602 participants (43.2% male) were analyzed. The population was consisted of relatively young individuals whose mean (± standard deviation (SD)) age was 43.89(± 15.36) years with median and interquartile rage (IQ) (25 - 75) of 42 and (32 - 55) years, respectively. Prevalence of CKD, defined by MDRD-based eGFR was 10.1% ( CI: 9.5, 10.7), whereas the age adjusted prevalence of CKD was 11.3% (CI: 10.7, 12.0). Prevalence of CKD, defined by EPI-based eGFR was 9.3 % (CI: 8.7, 9.9); while age adjusted prevalence of CKD was 8.5% (CI: 7.9, 9.1). Prevalence of CKD employing both equations was estimated to be higher in women than men. It was remarkable that the CKD-EPI equation determined CKD prevalence to be higher in men but lower in women. Using both equations, prevalence of CKD increased with age and was highest in those aged ≥ 70 years (Figure 1). In multiple logistic regression analysis, the multivariate-adjusted ORs for dyslipidemia, older age, female gender and hypertension were statistically significant with presence of CKD, by both equations; ORs of diabetes mellitus, smoking and abdominal obesity were not statistically significant in any of the CKD populations. In addition, OR of BMI > 25 was statistically significant in EPI-based CKD whereas it did not reach statistical significance in CKD population based on the MDRD equation.
3.2. Incidence
Using the MDRD equation, Tohidi et al. found that during a mean follow up of 9.9 years, the incidence density rates of CKD were 285.3 per 10000 person-years in women and 132.6 per 10000 person-years men. Female gender was linked with increased risk of developing CKD. Independent predictors for CKD in women comprised of age, being single or divorced/widowed, known diabetes, hypertension (marginally significant), current smoking and eGFR; conversely, education (intermediate degree) and family history of diabetes lowered the risk about 40% (P < 0.05). For men, risk factors of CKD comprised of age, hypertension (P for interaction comparing with women < 0.05), eGFR, newly diagnosed diabetes and high normal blood pressure. On the other hand, the risk of CKD was lowered by abdominal obesity about 30% (marginally significant). In Iran, more than 2% of population develop CKD annually (6).
3.3. Risk Factors
To evaluate the effect of body mass index, waist circumference (WC) and waist-to-hip ratio (WHR) on developing CKD in adults, a study by Noori et al. examined a representative sample of 3107 CKD-free subjects including (1309 men), aged > 20 years, and followed them for 7 years. Results showed that during follow-up, 419 participants (13.5%) developed CKD. After multivariable adjustment for a number of variables including age, sex, smoking, menopause, blood pressure, prevalent and incident diabetes, and change in WC, waist circumference was positively associated with risk of CKD. On the other hand, the WHR was not an independent risk factor of CKD. Interestingly, baseline waist categories affected the rate of decline in eGFR: Regression coefficient for 1 SD increase in WC was equal to -0.18 (CI: -0.28, -0.07). Accordingly, baseline WC was proved to be a better predictor of CKD than WHR (P < 0.05) or BMI (P < 0.05). The investigators concluded that comparing to WHR and BMI, waist circumference per se, was a more crucial determining factor of CKD risk in adults (7).
The relationship between chronic kidney disease and anthropometric measurements has been examined by several investigations, but the association between changes in waist circumference and incidence of CKD needs more clarification. In a study by Barzin et al., the effect of changes in WC on developing CKD was assessed in three consequent phases. A cohort of 8,183 CKD-free participants (46.5% men), mean age 47.4 years, were followed for 9 years. Four groups of waist changes were described: (I) Decrease in WC; (II) reference group; (III) mild to moderate increase in WC and (IV) severe increase in WC. Overall, mean MDRD based eGFR was higher in male participants (77.1 vs. 74.4 mL/min/1.73 m2 in women, P < 0.001). A total of 1477 participants (1026 women) developed CKD during the follow-up. Their results showed that in women, changes in WC were not correlated with CKD incidence. In men, decrease in WC was not correlated with decreased CKD incidence (Hazard ratio (HR) = 0.90, CI: 0.6, 1.4), however, group III was associated with a 70% augmented risk of CKD (HR = 1.6, CI: 1.2, 2.2). Category IV also, was linked to a fourfold risk of CKD (HR = 3.7, CI: 2.7, 5.1). The authors concluded that WC changes were not independent determinants for CKD occurrence in women. In men however, waist gain negatively affected the CKD incidence (8).
To explore the association of metabolic syndrome (MS) and risk for CKD occurrence independent of diabetes, Rashidi et al. studied a cohort of 4607 diabetes/CKD- free adults (age > 18 years) in the context of the Tehran lipid and glucose study. Their results showed that 1010 (21.9%) participants had MS at baseline. During the follow-up of 3590 individuals, CKD occurred in 3.4% (n = 38) of the MS group and 2.0% (n = 73) of the non-MS group (OR = 1.88, CI: 1.26, 2.8). Excluding participants who had hypertension at baseline (n = 798), 406 individuals (10.7%) fulfilled the MS criteria. CKD developed in 62 (1.82%) subjects in the metabolic syndrome group and 8 (1.98%) in the non-MS group (OR = 0.925, CI: 0.446, 1.917; P = 0.844). These findings proposed that metabolic syndrome, as a constellation of risk factors, was a significant predictor for CKD. However, hypertension significantly affected this relationship (9).
In another study, Derakhshan et al. evaluated the effect of various combinations of blood pressure status and glucose tolerance on the occurrence of chronic kidney disease, type 2 diabetes mellitus (T2DM) and hypertension. They included 12808 Iranian adults in 3 separate analyses to examine the incidence of each above mentioned disease. Multivariate Cox proportional hazard models were employed for the analyses. During follow-up (median > 10 years), the incidence rate for CKD was 24.8 per 1000 person-years. Every category that comprised of hypertension (HTN) and/or T2DM possessed significant risk of developing CKD. However, the pre-diabetes/HTN group showed a slightly significant risk (HR = 1.19; CI: 0.98, 1.43, P = 0.06) (10).
In another study, Ramezankhani et al., analyzed the correlation between CKD and hypertriglyceridemic waist (HW) and waist-to height ratio (HWHtR) phenotypes. The median follow-up was 12.4 years. A total of 12,012 participants in the TLGS phases 1 and 2 were analyzed. For prospective analysis, the data of 8225 individuals (45% men) were included. Outcome was defined as development of CKD (eGFR < 60 mL/min/1.73 m (2). The HW phenotype was described as waist circumference (WC) > 90 cm in men and > 85cm in women, in the presence of triglyceride (TG) > 2.0 mmol/L. The HWHtR phenotype was identified as waist-to-height ratio (WHtR) > 0.5 and TGs > 2 mmol/L. In cross sectional analysis, both the HW and HWHtR phenotypes were linked with CKD in women ((adjusted OR = 1.37, CI: 1.01, 1.86, P < 0.05) and (adjusted OR = 1.58, CI: 1.03, 2.41, P < 0.05)), correspondingly. Conversely in men, while in non-adjusted and age-adjusted models both phenotypes were correlated with CKD, the associations were lost in fully adjusted models. In prospective analysis, neither of the phenotypes were significant risk factors of developing CKD. The investigators concluded that while in a cross-sectional setting, HW and HWHtR phenotypes were correlated with prevalent CKD, in prospective analysis, HW and HWHtR did not significantly predict CKD (11).
To determine the effect of various obesity phenotypes on the CKD incidence in adults, in a prospective study, Mottaghi et al. observed that rates of CKD-free MHO (metabolically obese but healthy) and MONW (metabolically healthy normal weight) obesity phenotypes were 75.3% and 60.6%, correspondingly (P < 0.0001). In model 1 (adjusted for age and sex) hazard ratios of developing CKD in MHO or MONW obesity phenotypes were 1.14 ( CI: 0.91, 1.43) and 1.43 ( CI: 1.09, 1.88), respectively. In model 2 (comprising of further adjustment for confounders) hazard ratios of CKD incidence in MHO or MONW obesity phenotypes were 1.23 (CI: 0.93, 1.62) and 1.43 (CI: 1.08, 1.90), correspondingly. The authors concluded that comparing with people with the MHO obesity phenotype, risk of incident CKD was higher in adults with the MONW obesity phenotype (12).
In a recent longitudinal study aiming to evaluate the risk of incident CKD in adults with abdominal obesity, participants aged > 20 years from TLGS phase II were enrolled. Abdominal obesity was defined as waist circumference > 89 cm in men and > 91 cm in women. Metabolic health was defined as having ≤ 1 component of metabolic syndrome, employing the joint interim statement (JIS) definition of metabolic syndrome. The study observed that from among 6597 individuals who entered the study, 1529 participants were affected by CKD after 10 years of follow up. Multivariate regression models demonstrated that, compared to metabolically healthy non-abdominal obese participants, men with the metabolically unhealthy non-abdominal obese phenotype had a slightly significant risk of developing CKD (HR = 1.38, CI: 0.96, 1.96, P = 0.08). However no statistically significant difference was observed for metabolically healthy abdominal obese phenotype (Unpublished data).
3.4. CKD and CVD
Population based studies have revealed inconsistent findings concerning the association between CKD and CVD. To ascertain this association, the investigators examined the risk of CVD events in a sizable group of participants in the TLGS. A total of 6,209 CVD-free participants (mean age, 47.4 years) were followed up for 9.1 years. Among them, 22.2% (n = 1381) had MDRD based (eGFR < 60 mL/min per 1.73 m2) at baseline. Ninety nine percent of them were in stage 3a. After adjustment for age and sex, the results showed that moderate renal insufficiency independently predicted CVD outcomes. However, its statistical significance was lost (HR = 1.14, CI: 0.91, 1.42) after further adjustment. Moreover, after categorizing the participants according to CKD status and BMI groups, no significant association was observed after further adjustment (P = 0.2). This study concluded that CKD did not independently predicted events. The increased prevalence of CVD in subjects with mild to moderate renal insufficiency in the TLGS population might have been due to the presence of other known CVD risk factors in this group (13).
Another prospective cohort with longer follow up duration was conducted to investigate the independent role of CKD in predicting CVD events. In this population-based cohort the CKD-EPI equation was used to estimate GFR and define CKDs. For this study, phase II of the TLGS study was selected for baseline measurements. CKD was defined as eGFR < 60 mL/min per 1.73 m2. Of a total of 6185 participants with a median (IQ 25 - 75) follow-up of 10.2 (9.1 - 11.2) years, 3510 (56.8%) were women and 597 (9.7%) were diagnosed with CKD. The authors found that CKD was associated with future cardiovascular disease (CVD), especially in patients aged ≥ 55 years. This association was independent of well-known traditional CVD risk factors, i.e. hypertension, diabetes mellitus, hyperlipidemia, abdominal obesity, cigarette smoking, and family history of CVD. Furthermore, they reached a cutoff of 62 mL/min per 1.73 m2 for future CVD events in their population (Unpublished data).
It has been reported that there are some interactions between CKD and other metabolic disorders including metabolic syndrome and obesity regarding coronary heart disease (CHD) outcomes; to address these interactions, Panahi et al. followed a total of 2823 men and 3684 women, aged ≥ 30 years, without cardiovascular disease for 10 years. Multivariable adjusted hazard ratios of CHD were estimated for those who developed CKD, MS or both by sex and body mass index levels (below and above 27 kg/m2). In addition, interaction terms of CKD and MS and also CKD-MS components were assessed. Employing Cox proportional hazard models showed that chronic kidney disease without MS, had a significant effect on CHD only in participants with low body mass index (HR = 2.06; CI: 1.28, 3.31 and HR = 2.56; CI: 1.04 - 6.31 in men and women, respectively). In this subgroup, the combined effect of CKD and MS decreased to one-third of their multiplicative effect, showing that there was a negative interaction between CKD, MS, and obesity. Moreover, the same effect was spotted between CKD and hypertension in both sexes and CKD and type 2 diabetes mellitus in the men. The authors concluded that CKD independently predicted CHD only in non-obese individuals; however, its risk was vanished when joined to MS (14).
To examine the possible risk factors of stroke and their population attributable fraction (PAF), Fahimfar et al. designed a cohort study including 1089 men and 1289 women. The mean (SD) age for men and women was 61.1 (7.6) and 59.0 (6.7) years, respectively. Participants were followed for 9.3 years. To estimate the hazard ratio of each risk factor for stroke events Cox regression analysis with stepwise method was employed. A multivariate adjusted population attributable fraction (PAF) was calculated for any risk factors remaining in the model. During follow-up, stroke incidence rates was 4.5 ( CI: 3.3, 6.0) and 2.5 (CI: 1.7, 3.6) in 1000 person-years for men and women respectively. The results showed that age ≥ 65 years (HR = 2.03, CI: 1.24, 3.31), male gender (HR = 2.00, CI: 1.16, 3.43), hypertension (HR = 3.03, CI: 1.76, 5.22), diabetes mellitus (HR = 2.18, CI: 1.34, 3.56), and chronic kidney disease (CKD) (HR = 2.01, CI: 1.22, 3.33) independently predicted stroke events. They observed that hazard ratio of CKD did not differ from other independent risk factors as proved by paired homogeneity test. The PAFs were 29.7% and 25% for male gender and age ≥ 65 years and 48.6%, 29.1% and 22.0% for hypertension, CKD and diabetes, correspondingly. The authors concluded that among modifiable predictors, CKD as well as hypertension and diabetes are the strongest independent predictors of stroke (15).
Rashidi et al. in another investigation, tried to determine whether chronic kidney disease and diabetes mellitus- independent of hypertension- had similar prevalence of ECG abnormalities. Data for 5942 men and women aged 30 to 69 years in the TLGS were collected. Minnesota ECG coding criteria were employed for coding ECG findings. The authors implemented the Whitehall criteria for abnormal ECG findings. Creatinine clearance (CrCl) was estimated using the Cockroft-Gault equation. Comparison was made between DM free subjects with moderate CKD and CKD free patients with DM. HTN prevalence was comparable in both groups. The findings showed that despite a similar prevalence of smoking, and a lower incidence of dyslipidemia and HTN, more than 19% of patients with CKD showed abnormal ECG findings, while prevalence of abnormal ECGs in diabetic patients were 14.7% (P = 0.02). The prevalence of Q waves was 11.5% in patients with CKD and 10.8% in patients with DM. In an age-matched subgroup of CKD-free diabetic patients, the prevalence of ECG abnormalities was comparable to that of DM-free patients with moderate CKD (19.3% vs 19.7%, P = 0.9). This study concluded that moderate CKD is a key predictor for development of ECG abnormalities and hence, is linked with ischemic heart disease. The significance of CKD as a predictor of ECG abnormalities is similar to diabetes mellitus. Patients with moderate CKD are potential candidates for meticulous CHD risk reduction (16).
4. Conclusions
In this review, we examined the TLGS-based studies that have assessed different aspects of CKD during the last two decades. The topics addressed by reviewed studies included prevalence and incidence of CKD, effect of obesity and its different phenotypes, WC and its changes as well as metabolic syndrome and its components on CKD. Also role of CKD in developing CVD, metabolic syndrome and stroke and ECG abnormalities in CKD were examined.
One important strength of aforementioned studies is the fact that they examined sizable number of individuals in the context of a population based study (TLGS). However, their limitations merit further comment. Regarding CKD prevalence and incidence, in one study authors had not obtained data on urinary albumin and protein. Therefore in this population, the prevalence of stages I and II CKD could not be estimated. Also a single creatinine measurement was accepted for estimating eGFR; therefore one cannot ascertain the persistence of CKD for at least 3 months. Furthermore, serum creatinine measurements were not calibrated to the Cleveland Clinic; Also the MDRD eGFR equation was not validated in the local population, which could have caused an overestimation in the prevalence of CKD. Considering the association between obesity for CKD, a study pointed out probable misclassification of CKD due to using eGFR and the possible effect of greater muscle mass in individuals with higher BMI. Another study mentioned the overall young study population as its limitation for assessing the relationship between metabolic syndrome and CKD. And last but not least, studying the risk factors for ischemic stroke, one study listed different types of public and private healthcare systems as a barrier to detect all cerebrovascular events in district 13 of Tehran.
Results of the reviewed studies showed that age adjusted prevalence of MDRD-based and EPI-based CKD, were 11.3% (CI: 10.7, 12.0) and 8.5% (CI: 7.9, 9.1), respectively. Over a mean follow up of 9.9 years, the incidence density rates of MDRD based-CKD were 285.3 and 132.6 per 10000 person-year, among women and men, correspondingly. Assessing risk factors of CKD, studies conducted on the TLGS population documented that abdominal adiposity defined as waist circumference (WC) categories (P for trend < 0.02) and waist gain in men (HR = 1.7, CI: 1.3, 2.2), significantly affected development of CKD. With regard to the association between CKD and cardiovascular diseases, it was shown that CKD had a significant effect on coronary heart disease (CHD) only in participants with low body mass index (HR = 2.06; CI: 1.28, 3.31 in the men and HR = 2.56; CI: 1.04, 6.31 in the women). Moreover, it was reported that, CKD was amongst the strongest independent predictors of stroke (HR: 2.01, CI: 1.22 - 3.33). Also, compared to diabetic patients, moderate CKD was a main risk factor for the development of the abnormal ECG (P = 0.02).
In conclusion, the reviewed studies showed that in the TLGS population, increased waist circumference and waist gain (only in men) were associated with CKD development which was also an independent predictor of CHD (in lean individuals) and stroke.