1. Background
Hypophosphatemic rickets (HR) are rare diseases with an estimated incidence of 3.9 per 100,000 live births (1). In approximately 80% of all familial HR cases, the disease arises due to X-linked dominant inherited mutations in the PHEX gene located at Xp22.2-p22.1 causing an increase in fibroblast growth factor 23 (FGF23) levels (2). FGF23 regulates the phosphate homeostasis (3), and in HR, the increased FGF23 leads to an excessive renal loss of phosphate (4), which may result in rickets and/or osteomalacia, growth failure, and dental complications (5). Furthermore, increased FGF23 inhibits the enzyme 1α-hydroxylase, leading to an inappropriately low 1, 25-dihydroxyvitamin D (1,25(OH)2D) concentration (6). Less commonly, familial HR may be caused by an X-linked recessive inheritance (CLCN5) or an autosomal dominant or recessive inheritance (FGF23, DMP1, or ENPP1 mutation) (2, 7, 8). Alternatively, but extremely rarely, excess FGF23 is produced by tumors as in tumor-induced osteomalacia (TIO) (9).
HR is conventionally treated by a combination of oral phosphorus supplementation and vitamin D and/or vitamin D analogs. Careful titration is needed in order to treat rickets/osteomalacia, and avoid side effects (5). To maintain an appropriate level of plasma phosphate (P-phosphate), oral phosphate tablets should be administered four to five times daily, since the phosphate in tablets is rapidly absorbed and excreted (10). P-phosphate fluctuations with high peak concentrations may result in increased secretion of parathyroid hormone (PTH) resulting in secondary or eventually tertiary hyperparathyroidism, which further aggravates the phosphate excretion and bone resorption causing bone demineralization (11, 12); furthermore, nephrocalcinosis can occur as a consequence (13). Secondary hyperparathyroidism may necessitate treatment with cinacalcet to avoid constant high levels of PTH (14) leading to tertiary hyperparathyroidism.
The literature is sparse concerning the effect of different phosphorus sources on phosphate-calcium metabolic parameters in patients with HR. It is observed that an enhanced phosphorus content in food can disturb bone metabolism (15). A study by Karp et al. (16) on healthy females, evaluated the effect of equivalent phosphorus amounts given as meat, cheese, whole grain, or phosphate supplementation as a mixture on calcium and bone metabolism. They found the highest PTH concentrations during treatment with phosphate mixture. Furthermore, the urine phosphate (U-phosphate) was higher during the phosphate mixture treatment compared with those of the cheese and whole grain treatments. The results suggested that the phosphorus source might influence phosphate-calcium metabolism parameters.
To the best of authors’ knowledge, no studies evaluated the effect of different phosphorus sources on phosphate and calcium metabolism to treat HR.
2. Objectives
On the grounds of the results found by Karp et al. (16), the current study aimed at investigating the feasibility (patient compliance and satisfaction with treatment) and efficacy of treatment with equivalent phosphorus doses given as skimmed milk or cheese compared to phosphate tablets in patients with HR evaluated by P-phosphate, P-PTH, and U-phosphate excretion as the main effect parameters.
3. Methods
3.1. Participants and Protocol
Patients were selected from the outpatient clinics at Aarhus University Hospital (Denmark) from August 2015 to June 2016. All patients had genetically verified HR (Table 1) and were treated with oral phosphate tablets plus vitamin D (cholecalciferol and/or alfacalcidol). Patients were excluded from the study if they presented with tertiary hyperparathyroidism, were treated with cinacalcet, or had milk allergy or lactose intolerance.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Gender | Female | Female | Female | Female | Female | Female | Female |
| Age, y | 30 | 33 | 38 | 13 | 15 | 32 | 35 |
| Weight, kg | 88.4 | 76.2 | 80.6 | 38.0 | 52.2 | 70.2 | 93.2 |
| Height, cm | 159 | 157 | 159 | 132 | 137 | 156 | 180 |
| Inheritance | X-linked dominant | X-linked dominant | X-linked dominant | X-linked dominant | X-linked dominant | X-linked dominant | Autosomal dominant |
| Genetic variant | PHEX, c1103G>A | PHEX, c2239C>T | PHEX, c1587-1G>C | PHEX, c1587-1G>C | PHEX, c1587-1G>C | PHEX, pathogenic mutation | FGF23, c536G>A |
| P-creatinine at 1st visit, umol/L | 67 | 64 | 61 | 35 | 44 | 56 | 73 |
| 25OHD, nmol/L | |||||||
| Tablet | 62 | 47 | 43 | 41 | 33 | 42 | 45 |
| Milk | 59 | 53 | 50 | 43 | 41 | 35 | 43 |
| Cheese | 56 | 74 | 38 | 43 | 38 | 31 | - |
| 1.25(OH)2D (pmol/L) | |||||||
| Tablet | < 25 | 47 | < 25 | 53 | < 25 | 75 | 57 |
| Milk | < 25 | 38 | < 25 | 38 | 29 | 66 | 59 |
| Cheese | < 25 | 77 | < 25 | 48 | < 25 | 38 | - |
The current randomized (using: randomization.com), multiple crossover study had three treatment sessions consisting of ingestion of phosphate tablets, milk, or cheese with equivalent phosphorus content. Each treatment session lasted four days, and blood and urine samples were taken on the 4th day. The patients were instructed to discontinue their regular phosphate treatment and start the phosphorus substitution (phosphate tablets, milk, or cheese) three days prior to sample collection. Treatment with active vitamin D was continued throughout the study period and on the day of sampling. The patients were instructed to follow their normal dietary habits (amount and source of diet) while participating in the study; the normal diet was followed on the day of sampling as well. Patients fasted overnight before specimen collection. There was at least a four-day washout period between each treatment session.
When phosphate supplementation was gives in the form of tablets, the patients were treated with 800 mg of elemental phosphorus divided into five doses over the day, independent of any prior treatment dose. In the milk treatment session, all patients received 800 mL of skimmed milk divided into five meals corresponding to 800 mg of elemental phosphorus per day. In the cheese treatment session, all patients received the same kind of cheese with estimated elemental phosphorus content of 800 mg divided into five meals (18 g cheese per meal).
3.2. Sampling
On the 4th day of each treatment, the patients visited the clinic for the collection of blood samples taken at five time points during the day, including a fasting blood sample collected before any food or treatment at 8:00 a.m. Blood samples were collected at 8:00 a.m. (fasting), 10:00 a.m., 12:00 a.m., 2:00 p.m., and 4:00 p.m. All blood samples were analyzed for total P-calcium, P-phosphate, P-PTH, and P-FGF23; in addition, the fasting blood samples were analyzed for serum 25-hydroxyvitamin D (25OHD), serum 1,25(OH)2D, and P-creatinine. On the day of blood sampling, urine samples were also taken from the participants twice: 1) between 8:00 a.m. and 12:00 a.m., and 2) between 12:00 a.m. and 4:00 p.m. According to standard operating procedures, blood samples for batch analysis were centrifuged at 4000 rpm (3000 xg) for 10 minutes before being stored at -80°C until analysis. The urine samples were stored at -20°C until analysis. U-phosphate excretion was calculated as the U-phosphate concentration multiplied by the urine volume.
Except for the P-FGF23 analysis, all analyses were performed according to standard laboratory techniques at the Department of Clinical Biochemistry, Aarhus University Hospital. The 25OHD (reference range: 50 - 160 nmol/L) and 1,25(OH)2D (reference range: 60 - 180 pmol/L) were analyzed by liquid chromatography-tandem mass spectrometry, both with total assay coefficient of variation (CV) of 10%. PTH was analyzed using an immunometric assay (Cobas 6000, Roche Diagnostics Ltd., the UK) with total CV of 10%.
3.3. FGF23
C-terminal P-FGF23 concentrations were determined using a two-site enzyme-linked immunosorbent assay (ELISA), 2nd generation, from Immutopics Inc. (Human FGF-23 (C-Term) ELISA Kit; Cat. # 60-6100 96), following manufacturer’s instructions. Briefly, 100 µL of calibrators, controls, and EDTA (ethylenediaminetetraacetic acid) plasma samples were pipetted onto the streptavidin-coated plate. A 1:1 mixture of biotin- and HRP-labelled goat polyclonal antibodies directed against the c-terminal region of P-FGF23 was added to the samples and the plate was incubated for three hours at room temperature with gentle shaking (200 rpm). The plate was then washed five times with 350 µL of wash buffer and tetramethylbenzidine was added to the plates (150 µL) followed by incubation for another 30 minutes in the dark with gentle shaking (200 rpm). Absorbance was read at 620 nm before the development was stopped with 50 µL of stop solution. Absorbance was read again at 450 nm (reference at 620 nm). Absorbance at 620 nm was used for concentrations ranging 451-1400 RU/mL, while absorbance at 450 nm for concentrations below 451 RU/mL. The normal range in adults is < 100 RU/mL (17), intra-assay CV is 2.4%, and inter-assay CV is 4.7%. FGF23 concentrations were analyzed at Bioanalytical Facility, University of East Anglia, Norwich, the UK.
3.4. Statistical Analysis
The outcome variables including P-phosphate, P-PTH, total P-calcium, P-FGF23, and U-phosphate excretion were analyzed in a mixed model with nested random effects to account for the repeated observations on each subject and within-subject levels in the crossover design. The fixed part of the model included the treatment group and time interaction and was further adjusted for treatment number and treatment order (the row and column effect of the crossover design). Model validation was performed by graphical inspection of residuals, fitted values, and random effect estimates (BLUPs). P-FGF23 was analyzed using a log scale. Residual variation, i e, the variation between observations in the same subject and within the same treatment session, was group-specific and a test for difference in variation was performed by comparing this model to the model with equal variations by a likelihood ratio test. The hypothesis of parallel mean curves over time between the treatment groups was tested on the treatment-time interaction. Additionally, the overall group comparisons, computed as the hypothesis of parallel mean curves, did not seem implausible for any of the parameters. P-FGF23 was back transformed and comparisons were relative. All analyses were performed using Stata version 15 (IC) (StataCorp LLC, 2017. College Station, TX). P values < 0.05 were considered statistically significant.
3.5. Ethical Considerations
The Danish Ethics Committee and the Danish Data Protection Agency approved the study protocol; the study was also registered at ClinicalTrials.gov (NCT03348644). The patients were informed orally and in writing about the project and both oral and written consents were obtained before inclusion in accordance with the Declaration of Helsinki. Approval to enroll patients under the age of 15 years was obtained from legal guardians.
4. Results
Seven females, aged 13 - 39 years, were included. Six of the patients had dominant X-linked HR due to PHEX gene mutations, while one patient had dominant autosomal HR due to a mutation in the FGF23 gene. The characteristics of the participants are presented in Table 1. In the cheese treatment session, the patient with the autosomal dominant mutation did not participate and another patient did not give the blood sample at 4:00 p.m. In the phosphate tablets treatment session, one patient did not give the blood sample at 4:00 p.m. During the other sessions, all seven patients participated. The patients’ compliance was not registered, but all patients reported 100% compliance with the treatments. P-creatinine levels did not change during the treatment period. Three patients had low 1,25(OD)2D levels (Table 1).
Treatment feasibility was independent of the phosphorus source. One patient felt that the amount of cheese to be ingested was ample, but she was assured and compliance was 100%. None of the patients reported difficulties with drinking extra 800 mL of milk daily.
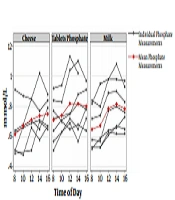
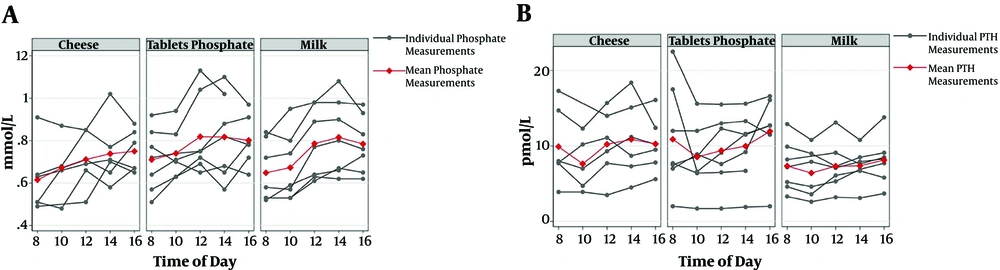
Mean P-PTH was significantly lower during the treatment with milk compared to phosphate tablets (P < 0.05) (Table 2). However, P-phosphate, P-FGF23, and total P-calcium levels did not differ significantly among the treatments. Figure 1 presents the P-phosphate and P-PTH levels by the time of day in the three treatment modalities. U-phosphate excretion between 8:00 a.m and 4:00 p.m. was significantly lower during the milk treatment session than that of the phosphate tablets (P < 0.05) (Table 2).
| Milk vs. Tablet, Estimate (95% CI) | Cheese vs. Tablet, Estimate (95% CI) | |
|---|---|---|
| P-phosphate, mM/L | -0.04 (-0.13; 0.04) | -0.07 (-0.17; 0.02) |
| P-PTH, pmol/L | -2.71b (-4.56; -0.86) | 0.01 (-2.03; 2.05) |
| P-FGF-23c, RU/mL | 1.00 (0.73; 1.36) | 0.80 (0.58; 1.12) |
| Total P-calcium, mM/L | 0.03 (-0.03; 0.09) | -0.04 (-0.11; 0.02) |
| U-phosphate excretion, mM/L | -1.79b (-3.23; -0.35) | -1.39 (-2.94; 0.17) |
aP-PTH and U-phosphate excretion was significantly lower during the treatment with milk compared to phosphate tablets.
bP values < 0.05.
cAnalyses were performed based on log-scale. Comparisons are relative.
A significant variation was observed in P-phosphate and P-PTH fluctuations when comparing the three treatment modalities (P < 0.01) and a significantly lower variation was observed when administering phosphate as milk compared to phosphate tablets (P < 0.05) (Table 3 and Figure 2). The same trend was observed in the cheese treatment session, although not statistically significant. There was no significant difference in P-FGF23, total P-calcium, or U-phosphate excretion when evaluating the fluctuations.
| Tablet | Milk | Cheese | P Value | |
|---|---|---|---|---|
| P-phosphate, mM/L | 0.08 (0.06; 0.1) | 0.04 (0.03; 0.05) | 0.07 (0.05; 0.09) | < 0.01 |
| P-PTH, pmol/L | 2.51 (1.89; 3.34) | 0.92 (0.69; 1.22) | 1.56 (1.13; 2.14) | < 0.01 |
| P-FGF-23b, RU/mL | 0.08 (0.06; 0.11) | 0.11 (0.08; 0.15) | 0.10 (0.07; 0.13) | 0.32 |
| Total P-calcium, mM/L | 0.04 (0.03; 0.06) | 0.04 (0.03; 0.05) | 0.03 (0.02; 0.04) | 0.21 |
| U-phosphate excretion, mM | 2.09 (1.36; 3.2) | 0.92 (0.46; 1.85) | 1.29 (0.75; 2.22) | 0.06 |
aP values compared the variation in the three groups.
bAnalyses were performed based on log-scale.
5. Discussion
The current study demonstrated reduced P-PTH and reduced renal phosphate excretion and furthermore, reduced fluctuations in P-phosphate and P-PTH when administrating phosphate substitution to patients with HR as milk compared to tablets. To the best of authors’ knowledge, it was the first study reporting the effect of different phosphorus sources when treating HR.
There is a known biological interaction between P-phosphate and P-PTH. The increase of P-PTH leads to increased phosphate excretion when P-phosphate is high. Decreased P-PTH leads to reduced phosphate excretion when P-phosphate is low (18). P-phosphate fluctuations with high peak concentrations, possibly in combination with reduced calcium concentrations, may be observed in response to treatment with phosphate tablets leading to increased P-PTH concentrations (12). The observation of lower P-phosphate fluctuations when administering the phosphorus source as milk combined with the reduced P-PHT and reduced renal phosphate excretion is thought to decrease the long-term risk of development of secondary and tertiary hyperparathyroidism (12). Greater urine phosphate excretion during the period on tablets could indicate that more phosphorus is absorbed from tablets than from milk or cheese, or it may be due to the lower P-PTH during the milk treatment session. However, P-phosphate did not differ significantly among the treatment periods, suggesting that the lower P-PTH during the milk session caused the lower phosphate excretion. There was no significant difference in P-FGF23 levels or fluctuations in P-FGF23. It was, however, expected since X-linked dominant, inherited mutations, as well as autosomal dominant inheritance cause increased levels of P-FGF23 independent of the P-phosphate concentration (18). Three patients had very low levels of 1,25(OH)2D (Table 1), and the other four patients had 1,25(OH)2D in the lower normal range. This may also be expected since P-FGF23 decreases the expression of the 1α-hydroxylase, thereby, inhibiting the hydroxylation of 25OHD to 1,25(OH)2D (2).
Karp et al. (16) investigated the acute effect of different phosphorus sources (meat, cheese, whole grains, and phosphate supplement as phosphate mixture) on calcium and bone metabolism in 16 healthy females aged 20 - 30 years. They found that cheese increased the P-phosphate concentration more than the other phosphorus sources when given in equimolar phosphorus quantities. P-phosphate concentration remained significantly higher the following morning. Phosphate mixture increased U-phosphate excretion more than whole grain or cheese. It was in line with the current study findings, though not significant. Karp et al. (16) found that the S-PTH concentrations differed among the different study sessions. Cheese was associated with the greatest decrease in S-PTH compared to all other phosphate sources. In contrast, phosphate mixture increased S-PTH concentrations compared to the control session. Meat or whole grain had no effect on S-PTH (16). Also, an insignificant trend of reduced P-PTH fluctuation and reduced U-phosphate excretion was observed when ingesting phosphorus as cheese. It was speculated that the reduction in P-PTH fluctuations when ingesting phosphorus as milk or cheese was caused by prolonged phosphorus absorption. However, the exact mechanism remains to be elucidated.
In the future, human anti-FGF23 antibody may become an alternative treatment for HR caused by elevated P-FGF23 (19, 20). However, phosphorus supplements are still expected to be a necessary treatment option in HR as human anti-FGF23 antibodies might not be available in all countries, and their efficacy over time needs further verification. Furthermore, milk as phosphorus source may be an alternative to phosphate tablets in other phosphate wasting diseases such as renal tubular acidosis or TIO (21).
Although the low number of the studied patients was a limitation of the study, it was a strength point that significant differences were demonstrated on the effect of parameters in the three treatment arms. Hereditary HR is a rare disease leaving difficulties in recruiting a larger study population. Furthermore, several patients in the current study received cinacalcet treatment, and were thereby excluded. Other limitations were the duration of the study, which only provided short-term information on the biochemistry, and the fact that there was no information regarding the phosphate content in the patients’ regular diet during treatment interventions. However, the patients were instructed to follow their usual dietary habits regarding both the amount and source of food.
Participants were only females, but no difference is expected in the pathophysiological mechanism of phosphate processing between males and females.
5.2. Conclusions
The current pilot study showed that the phosphorus supplement in patients with HR when given in equimolar phosphorus doses could be administered as phosphate tablets, milk, or cheese with similar phosphorus concentrations independent of the source. However, mean P-PTH level and U-phosphate excretion were significantly lower during treatment with milk compared to treatment with phosphate tablets. Furthermore, reduction of P-phosphate and P-PTH fluctuations after ingesting phosphorus as milk was statistically significant, suggesting that milk is less likely to be associated with unwarranted effects on the calcium-phosphate homeostasis compared to phosphate tablets in patients with HR. Since the study population was small and the differences in the effect parameters among treatments were also small, though statistically significant, the current study results need to be confirmed in a larger population.