1. Context
Polycystic ovary syndrome (PCOS) is a prevalent endocrinopathy in females (1), characterized by chronic oligo-anovulation, hyperandrogenism (HA), and polycystic ovaries (2), all of which can result in worsening of quality of life for these patients (3, 4). Precise prevalence of PCOS in adolescents is unknown, yet a recent study of females, aged 15 to 19 years old estimated it to be 1.14%, using NIH criteria (5). In addition to reproductive consequences, PCOS can be associated with a wide range of cardio metabolic disorders, including obesity, type 2 diabetes mellitus, dyslipidemia, metabolic syndrome, hypertension, and risk factors for cardiovascular disease (6-9).
Adolescence is a transitional stage of physical and psychological development, generally occurring during the period between puberty and adulthood; functional variations in the hypothalamic-pituitary-ovarian axis during normal puberty leads to changes in reproductive hormones and menstrual patterns that mimic some of the features of PCOS, complicating the diagnosis of PCOS in adolescent female populations (10). In other words, signs and symptoms of PCOS overlap the physiologic changes of puberty (11). Using adult diagnostic criteria for adolescents with suspected PCOS has always raised concerns about mis- or over diagnosis in this age group (1, 12).
Since in patients with PCOS, the main clinical problem is the control of menstrual irregularity cycles and hirsutism (13, 14), Oral Contraceptive (OCs) may ideally be used, with the usual contraindications to administration carefully considered. Lifestyle modifications should be used in obese adolescents; in some patients, especially if glucose intolerance is present, metformin may be added. Antiandrogen treatments should be recommended for hirsutism if there is no improvement following hormonal treatment (13). However, there are limited data on whether any treatment may be useful for improving PCOS manifestations in adolescence towards adulthood (14).
Although early identification and management of adolescents with PCOS can prevent the long-term reproductive, cardio-metabolic, and emotional consequences associated with syndrome in their future life (12, 13, 15), over diagnosis can also influence an adolescents’ quality of life and create an early and unwarranted anxiety about future fertility (15-17).
According to recommendations of the European Society of Human Reproduction and Embryology/The American Society for Reproductive Medicine (ESHRE/ASRM) sponsored PCOS consensus workshops, there are knowledge gaps regarding various aspects of PCOS in adolescents, viz. absence of longitudinal studies during adolescence, absence of specific diagnostic criteria for identifying PCOS during this period, and absence of normative values for a number of biochemical markers, and lack of clarity as to whether the severity of symptoms through this stage predicts the extent of the disorder in later life (14).
Because of the paucity of data focusing on PCOS in adolescence and the existence of important gaps and controversies regarding the recognition and management of this syndrome in adolescent females, the current researchers aimed at summarizing challenges and recommendations of diagnosis and treatment in this age group.
2. Evidence Acquisition
This review summarizes all papers published in the context of PCOS in female adolescents. PubMed, Scopus, Web of Science, and Google scholar databases were searched for retrieving reviews, clinical trials, and observational studies on PCOS in this group up to March 2019. The final selection of papers was made based on their relevancy with fields of diagnosis and treatment of PCOS in adolescent females.
3. Results
3.1. How to Diagnose Polycystic Ovary Syndrome in Adolescents
Although consensus on the application of determined diagnostic criteria in adults include NIH (National Institute of Health), Rotterdam, and AES (androgen excess society) criteria (Box 1) (1), these criteria are somewhat controversial when used for adolescents (1). In fact, the diagnostic pathologic features used in adults, viz. menstrual irregularity, hirsutism, acne, and polycystic ovary morphology (PCOM) may be common physiologic changes of puberty in normal adolescent females (1, 12). In this regard, Amsterdam criteria suggests that all three elements of the Rotterdam criteria should be present in teenagers for diagnosis of PCOS (14). The Endocrine Society clinical guidelines suggest the use of NIH criteria, including HA and persistent anovulatory menstrual cycles for diagnosis of PCOS in adolescents (18). Since there was not enough evidence to confirm the NIH criteria for adolescents, the Pediatric Endocrine Society recommended the criteria of persistent HA and oligo-anovulatory cycles based on suitable standards (Box 2).
| Diagnostic Criteria |
|---|
| NIH (1990) criteriab |
| Ovulatory dysfunction |
| Clinical hyperandrogenism and/or hyperandrogenemia |
| Rotterdam ESHRE/ASRM Consensus Conference 2003c |
| Oligoovulation or anovulation |
| Clinical and/or biochemical signs of hyperandrogenism |
| Polycystic ovaries on ultrasound |
| Androgen Excess Society Criteria (2006)b |
| Hyperandrogenism: Hirsutism and/or hyperandrogenemia |
| Ovarian dysfunction: oligo-anovulation and/ or polycystic ovaries |
Abbreviations: NIH, National Institutes of Health; PCOS, polycystic ovary syndrome.
aNote: For all diagnostic criteria, PCOS is diagnosed after exclusion of other disorders including non-classic adrenal hyperplasia, androgen secreting tumors, hyperprolactenemia, thyroid disorders.
bRequired both criteria to make a diagnosis of PCOS.
cRequired at least two criteria to make a diagnosis of PCOS.
| Diagnostic Criteria |
|---|
| Amsterdam criteria (2012)a |
| Oligoovulation or anovulation |
| Clinical and/or biochemical signs of hyperandrogenism |
| Polycystic ovaries on ultrasound |
| Endocrine Society criteria (2013)b |
| Oligomenorrhea |
| Clinical hyperandrogenism and/or hyperandrogenemia |
| Pediatric Endocrine Society criteria (2015) |
| Abnormal uterine bleeding pattern |
| a. Abnormal for age or gynecologic age |
| b. Persistent symptoms for 1 - 2 years |
| Evidence of hyperandrogenism |
| a. Persistent testosterone elevation |
| b. Moderate-severe hirsutism is clinical evidence of hyperandrogenism |
| c. Moderate-severe hirsutism or moderate-severe inflammatory acne vulgaris |
aRequired all three Rotterdam criteria to make a diagnosis of PCOS in adolescents.
bRequired both NIH criteria to make a diagnosis of PCOS in adolescents.
3.1.1. What is the Evidence of Oligo-Anovulation in Adolescents?
Many young females experience degrees of menstrual irregularity during their adolescence, especially in the first two years after menarche, due to higher frequency of anovulatory cycles (12, 19). The 5th and 95th percentiles for cycle length within the first gynecologic year are 18.3 and 83.1 days (12). Previous studies on variation of the menstrual cycle length showed that maturation of the hypothalamic-pituitary-ovarian axis can take up to five years after menarche (20, 21), whereas recent studies reported that regular menstrual patterns can be established within 6 to 12 months of menarche (22-24). Generally, there is a misconception that any degree of amenorrhea or menstrual irregularity during adolescence is normal and result of the natural process of puberty, whereas it should be considered that menstrual cycles of adolescent females differs only slightly from those of their aged counterparts (1). Endocrine society clinical guidelines suggest that an abnormal menstrual bleeding due to anovulation, if persistent, is a matter for concern (18). The precise definition of persistent oligo-anovulation in adolescents is yet imprecise for diagnosing PCOS (12), with some investigators suggesting that this finding should be present for at least two years after menarche, or primary amenorrhea at the age of 16 years (11). Since the risk of anovulation is greater in adolescents with HA than in their non-HA counterparts, females with anovulation manifestations should be evaluated for HA (25). Indeed, menstrual cycles intervals < 20 or > 90 days are abnormal even in the first post-menarcheal year and require further investigation (12).
3.1.2. What Is the Clinical Evidence of Hyperandrogenism in Adolescents?
There is no agreement on the clinical criteria of HA in adolescent females (26). However, hirsutism, and moderate to severe acne in adolescent females should be considered as the clinical manifestations of HA (12, 26, 27). Alopecia is rare during adolescence and is seldom considered as a diagnostic criterion (28). Comedonal acne is common in adolescent females, whereas moderate to severe inflammatory acne is scarce during puberty (29). Because systemic medical treatment may mask HA, patients with persistent acne undergoing topical treatments, or those with moderate-severe inflammatory acne should be assessed for HA before the initiation of these treatments (1, 12).
3.1.3. What Is the Biochemical Evidence of Hyperandrogenism in Adolescents?
Diagnosis of biochemical HA in young PCOS patients, particularly in adolescence, requires reliable tests with well-defined normal ranges (1, 15, 27, 30). Measuring total and free testosterone markers, as major serum androgens (30), has been proposed to initiate the evaluation of HA (27). Sex Hormone Binding Globulin (SHBG) serum concentrations may be a valuable marker to detect free androgen serum concentration (15). Measuring androstenedione (A4) and Dehydroepiandrosterone Sulfate (DHEAS) is commonly recommended to evaluate adrenal HA (3, 31, 32). A recent meta-analysis confirmed the relationships of A4 and DHEAS with FG (Ferriman Gallwey) score, making these parameters useful for evaluating hirsute patients with PCOS (3). Puberty changes can confound the diagnostic criteria of hyperandrogenemia in adolescents (12). However, it should be kept in mind that shortly after menarche, testosterone levels increase during puberty to reach peak approximating adult levels that make it a valuable androgenic marker for diagnosing hyperandrogenemia (1). In adolescent females with anovulatory menstrual cycles, testosterone levels are often increased (1, 14). Persistent elevation of serum total and/or free testosterone levels by > 2 SD above the mean of adult norms, determined by a reliable reference laboratory, can be considered a valid criterion for HA diagnosis in an adolescent female with PCOS signs, in whom, elevated androgen levels alone are not enough to detect HA, unless they have persistent hyperandrogenemia and anovulation (1, 33).
3.1.4. How to Diagnose Polycystic Ovary Morphology in Adolescents?
There are controversies in the diagnosis of PCOM in adolescents, mainly due to the high prevalence of physiological changes in their ovaries (34, 35). Hence, ultrasound is not a first-line investigation in adolescents; instead, their ovarian dysfunction should be detected based on oligomenorrhea and/or biochemical evidence of oligo/anovulation (15). Recent studies showed features of PCOM in a large population of normal adolescents (36-38). In fact, the ovary volume begins to increase with the onset of puberty, reaches the maximum volume between menarche and the age of 16, and remains stable or decreases after this point (12). It should be also considered that adult PCOM criteria become unreliable when applied to adolescents, especially when using transabdominal ultrasonography follicle counts in virginal adolescents (38). An ovarian volume of > 12.0 cm3 can be recommended for identifying PCOM in this age group (1, 36). In general, PCOM, in the absence of other diagnostic criteria, cannot predict the progression of PCOS and physicians should be cautious as this may cause anxiety due to possible inaccurate interpretation of ultrasound results for adolescent females and their families (34).
3.1.5. What Diagnostic Procedures Are Appropriate in Adolescents to Exclude Other Causes of Hyperandrogenism and Amenorrhea?
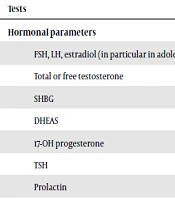
According to PCOS criteria, its diagnosis requires exclusion of other causes of HA and amenorrhea (1). A precise medical history, physical examination, and appropriate laboratory assessment are needed to exclude other disorders associated with HA (24). The initial workup often includes serum gonadotropins Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH), total testosterone, free testosterone, SHBG, DHEAS, and an early morning serum 17- hydroxyprogesterone level. Based on medical history and physical examination, additional evaluations may be done (39). Since Non-Classic Congenital Adrenal Hyperplasia (NCCAH) is the most important disorder in the differential diagnosis of PCOS, all guidelines recommend screening for this disorder (12, 18). Screening for hypothyroidism is strongly recommended because this disorder can result in irregularity of menstrual cycles and thickening of the hair (40). Guidelines support screening all patients with HA for hyperprolactinemia since it was reported in 16% of teenage females with PCOS characteristics (41). Box 3 summarizes the common laboratory tests for adolescents with PCOS-Like symptoms.
| Tests |
|---|
| Hormonal parameters |
| FSH, LH, estradiol (in particular in adolescents with amenorrhea) |
| Total or free testosterone |
| SHBG |
| DHEAS |
| 17-OH progesterone |
| TSH |
| Prolactin |
| Metabolic parameters |
| FBS |
| Fasting insulin |
| Lipid profiles (TG, TC, LDL-C, HDL-C) |
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; FBS, fasting blood sugar; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LH, luteinizing hormone; PCOS, polycystic ovary syndrome; SHBG, sex hormone binding globulin; TC, total cholesterol; TG, triglyceride; TSH, Thyroid-stimulating Hormone.
3.1.6. What Is the Role of Insulin Resistance/Hyperinsulinemia and Metabolic Syndrome in the Diagnosis of Polycystic Ovary Syndrome in Adolescents?
Insulin resistance/hyperinsulinemia are specific findings in PCOS and occurs both in obese and lean women (42), and may play a major role in the pathogenesis of PCOS (12, 16, 42). The author’s previous meta-analysis demonstrated associations between insulin levels and androgens in reproductive aged women with PCOS (43). Hyperandrogenemia and insulin resistance are related to metabolic syndrome in PCOS (42), and it has been suggested that PCOS may be a specific finding of the metabolic syndrome in females (44). Recent meta-analyses confirmed an increased risk of metabolic syndrome in adolescents with PCOS compared with healthy adolescent controls (7, 45). Although insulin resistance and obesity are usually associated with PCOS and exacerbate the HA and metabolic manifestations of PCOS in adolescents, these metabolic disorders should not be used as diagnostic criteria of PCOS among adolescent females (1, 12). However, in the presence of obesity, metabolic syndrome or manifestations of insulin-resistant, physicians should consider the possibility of PCOS and its related comorbidities, including metabolic syndrome (1, 45).
3.1.7. What Are the Risks of Over/Incorrect Diagnosis of Polycystic Ovary Syndrome in Adolescence?
Although a timely diagnosis of PCOS leads to awareness of this lifelong condition and provides an opportunity for therapeutic interventions, such as lifestyle modifications, assessing for HA, and its comorbidities, or medications (46, 47), it should be considered that over/incorrect diagnosis of PCOS can lead to unnecessary diagnoses and unwarranted interventions. Over diagnosis can also influence the adolescent quality of life and create unwarranted anxiety about future fertility for the young women and their families. Hence, using reliable diagnostic criteria, re-evaluation of the all adolescent females with probable PCOS (16, 17) is needed to avoid over/incorrect diagnosis and unnecessary treatment in healthy normal females without HA (12).
3.2. What Treatment Approaches Are Recommended for Adolescents with Polycystic Ovary Syndrome?
In patients with the diagnosis of PCOS, the main clinical problem is the control of menstrual irregularity and hirsutism (14); hence, approaches of treatment for an adolescent with PCOS are primarily directed at the major clinical manifestations and complaints (10, 18). Several treatment options have been developed for each of these, and some options address more than one symptom (13), as summarized in Box 4. Lifestyle modification is mainly considered as the first-line non-pharmacological treatment for adolescents with PCOS (10). Pharmacological agents include OCs, anti-androgens and metformin, used separately or combined (10, 33).
| Options |
|---|
| Lifestyles interventions |
| Weight loss |
| Physical exercise |
| Nutrition modifications |
| Combination of weight loss, physical exercise, and nutrition modifications |
| Local therapies |
| Laser |
| Electrolysis |
| Other methods |
| Metformin |
| Antiandrogens |
| Spironolactone |
| Flotamid |
| Finasteride |
| Oral contraceptives |
| Products with low androgenic effects |
| Products with antiandrogenic effects |
| Combination therapy |
Abbreviation: PCOS, polycystic ovary syndrome.
3.2.1. Lifestyle Intervention
Most patients with PCOS are obese. Regardless of BMI, patients with PCOS present intrinsic insulin resistance that exacerbates with overweightness or obesity (18, 48). Obesity worsens the clinical features and the endocrine profiles and obese females should hence be encouraged to lose weight; they should also be evaluated for fasting glucose tolerance and fasting insulin (49). In fact, weight reduction through restricting caloric intake and increasing physical exercise can be effective in regulating menstrual cycles (18, 48). A systematic review showed that lifestyle modification as the first-line of treatment in young patients with PCOS can improve their clinical, hormonal, and metabolic findings. This review concluded that calorie restriction and weight loss can directly improve disease outcomes in PCOS patients, although the effect of diet composition has not yet been well- elucidated (48).
3.2.2. Local Therapies
Physical approaches as non-pharmacological options to remove direct unwanted hair, including epilation, laser, or electrolysis may be acceptable to many patients and should be discussed with them (13, 14); these interventions can significantly improve quality of life scores (50). Since medical therapies may take at least six to nine months before any benefit is perceived, local therapies by laser, electrolysis, waxing, and bleaching may be helpful in the interim period. In cases with moderate to severe hirsutism, combination of cosmetic and medical therapies can be recommended (49). Severe acne can be treated with isotretinoin, although improvements may differ; this drug is not effective for hirsutism and may sometimes lead to alopecia (14).
3.2.3. Metformin
Using metformin in PCOS patients is still controversial and is approved only for an abnormal glucose tolerance test (51). Metformin is commonly used in young females and adolescents with PCOS as first-line medication with or without combination with OCs and anti-androgens (52). This drug can regulate menstrual cycles and decrease hyper-androgenemia through improving insulin sensitivity (53, 54). However, it should be considered that in adolescents, who cannot receive OCs, especially in those with obesity and abnormal glucose tolerance tests, metformin may improve symptoms (15). In lean adolescent females, a daily dose as low as 850 mg may effectively improve PCOS symptoms, whereas in overweight and obese adolescents, the dose should be increased to 1.5 to 2.5 g (42).
3.2.4. Oral Contraceptives
Given that regulating menstruation and reducing manifestations of hyperandrogenemia is the priority for any adolescent with PCOS, OCs can be the first line of medical treatment for most adolescents (55, 56), especially for those with hirsutism (56). The researcher’s recent meta-analysis showed that in patients with PCOS, OCs can improve biochemical and clinical HA. Although different products have almost similar effects on the hormonal profiles of patients, OCs containing Cyproterone Acetate (CPA) had more effect on hirsutism (57). Generally, OCs, whether an antiandrogen or not, may be safely used, keeping in mind the usual contraindications to administration (14). Worsening carbohydrate metabolism and lipid profiles with OCs in PCOS is an important issue for long-term adverse metabolic and cardiovascular effects (58); OCs containing less androgenic progestin may have less deleterious effects on insulin resistance and lipid profiles (13). The researcher’s previous meta-analysis showed significant elevations in lipid profiles of reproductive age women with PCOS; all OCs studied had similar effects, while products containing CPA generally required more time to increase serum lipids (59). Due to insufficient studies conducted on adolescent patients, a definitive conclusion about their long-term risks and safety, especially about breast cancer and cardio-metabolic disorders cannot be drawn (13, 60).
3.2.5. Antiandrogens
Antiandrogen treatments should be recommended for hirsutism when six to nine months of hormone therapy has failed; these products improve hirsutism by decreasing androgen production and binding the androgen receptors in target tissue (56). Generally, two types of antiandrogens are used for managing PCOS: Androgen receptor blockers, such as spironolactone and flutamide, and 5α-reductase inhibitors, such as finasteride (15, 33). Spironolactone is the most commonly used antiandrogen therapy in adolescents with PCOS. Regarding the risk of teratogenicity with antiandrogens, if pregnancy occurs and/or erratic menstrual bleeding from using spironolactone at a dose of 50 to 200 mg daily, it is recommended to use it in combination with OCs (49, 56). Although flutamide is not available in some countries because of its probable hepatotoxicity effects at high doses, evidence shows that use of 1 mg per kg daily is effective and not hepatotoxic, even with extended use (61). A main concern of anti-androgen therapy in adolescents is the effect on Bone Mineral Densitometry (BMD) (15, 62). However, there is a paucity of literature on use of these medications in adolescent populations and the methodological quality of studies available has been low (15, 33).
3.2.6. Combination Therapy
The aim of combined treatments is to improve PCOS symptoms through the additive or synergic effects of multiple therapies (33). Lifestyle modifications are baseline interventions for most adolescent females with PCOS, particularly those with overweightness or obesity, which can be added to first line treatments, such as OCs, metformin, or antiandrogens (14, 33). Combing metformin with OCs and lifestyle modification in obese PCOS patients could be considered if OCP and lifestyle changes do not achieve the desired goals (14, 51). Combination of OC and antiandrogens should only be considered to improve androgen related alopecia, or improve hirsutism when OC and cosmetic therapy for ≥ six months have failed to improve symptoms (14); in addition, in sexually active adolescents, OCs should be added to the treatment to prevent unwanted pregnancy (49).
3.2.7. Other Treatments
Regarding alternative treatments, such as orlistat and inofolic used for patients with PCOS, there is inadequate evidence on the efficacy of these remedies in improving PCOS manifestations (63, 64). There is also lack of strong evidence available demonstrating the effects of other alternative treatments, such as kinesiology, herbalism, homeopathy, reflexology, acupressure, acupuncture, or massage therapy (65).
4. Conclusions
This review underscores the use of standard diagnostic criteria for PCOS developed for adolescents. All adolescent females with persistent HA and oligo-anovulation should be assessed for PCOS. Although early recognition and management in these girls with PCOS can prevent the long-term reproductive, metabolic, and psycho-emotional complications associated with this syndrome, clinicians should re-evaluate all such patients with features very similar to PCOS to avoid over/incorrect diagnosis using precise criteria suggested for this age group. Appropriate therapeutic options for adolescents with PCOS should be recommended based on the major clinical manifestations and complaints.