1. Background
Methamphetamine (MA) is a highly addictive and psychostimulant drug. Its basic composition and structure are similar to those of amphetamine (C9H13N) (1-3). The chemical effects of MA are more than those of the amphetamine. MA is a white and crystalline powder with bitter taste. It is prescribed by physicians to treat attention-deficit/hyperactivity disorder (ADHD); nowadays due to its high potential for abuse as well as addictive and stimulant effects, medical uses of MA are limited (4).
In recent years, due to ease of production and low price, MA consumption has increased among the abuser populations, compared to cocaine and heroin (5). Over 35 million people use MA worldwide, whereas according to the United Nations office on drug and crime, only about 15 million subjects are heroin users and 10 million cocaine users (1, 6). Another reason for more popularity of MA abusing compared to other stimulant substances is its longer half-life (8 to 24 hours), compared to the cocaine (1 to 3 hours) (7).
MA addictive effects are among the major public health problems (6, 8). Population-based studies reported that 16% of young people within the age range of 20 to 29 years are the MA abusers (9). Nowadays in North America, MA is the most common addictive synthetic drug (10). Among European countries, the highest rate of MA consumption was reported in Czech Republic, Slovakia, and Hungary (11, 12).
MA has expanded deleterious effects on physical, psychological, and cognitive activities. Generally, there are 2 categories of short-term and long-term MA-induced symptoms. Short-term effects of MA abuse are euphoria, increased libido, increased energy, alertness, hyperactivity, and sense of well-being (13). Following the euphoria, which is the main reason for tendency toward MA abuse, the irritability is raised and in some individuals it can lead to aggressive behaviors (14). MA long-term abuse can cause serious psychological complications such as intense paranoia, violence, visual and auditory hallucinations, and delusion (3, 14, 15). Other MA side effects include cardiovascular disorders, hyperthermia, decreased appetite, insomnia, seizure, epistaxis, extreme weight-loss, nausea, vomiting, severe dental problems (meth mouth), losing teeth, gum disease, stroke, muscle cramps, and tremor (14). Important issue about MA chronic use can remain psychological complications such as depression, anxiety, and paramnesia even after years of pulling out of its use (4, 16). Therefore, it seems that MA consumers, compared to abusers of other addictive materials, face more and various psychological disorders (17). The first effect of the long-term MA abuse is an addiction that is probably accompanied by chemical and molecular changes in the brain. MA abuse causes release of neurotransmitters including dopamine (principally), norepinephrine, and serotonin (18, 19). Dopamine (DA) releases from vesicular storage sites into the cytoplasm (20), following the MA consumption and can cause increment of its cellular by products that in turn may lead to DNA damage (21) and produce neurotoxic quinones; production of reactive oxygen species (ROS) causes neurotoxicity (22), apoptosis induction, and cell degeneration (23). In the next step, motor and psychological impairments are occurred due to the cellular loss in hippocampus and striatum (24). MA dependency also raises the risk of susceptibility to infectious diseases such as HIV and hepatitis B and C viruses (25).
Neuroimaging studies in MA consumers showed structural abnormalities in their central nervous system (CNS). In addition, magnetic resonance imaging (MRI) investigations also revealed 3 key changes in the brain structure of MA consumers (4, 26); reduction of the gray matter volume in the limbic system and cingulate gyrus, explicit hypertrophy in the white matter of temporal lobe (especially around hippocampus), and reduction of hippocampus volume (27). Some volumetric studies also showed that MA abuse can cause reduction of volume in striatum (28), hippocampus (29), basal ganglia and cerebellum, and increment of cortical gray and white matters of some areas of the brain (30, 31).
The information obtained from MR images can be used to understand the precise structural changes, and volumetric and quantitative morphometric assessment of substance abusers over the time (32). Stereology is a branch of applied mathematics that yielded quantitative and 3-dimensional (3D) estimations of volume, area, length, and number from 2 dimensional slices of an object (33, 34).
2. Objectives
The current study aimed to evaluate the stereological changes of the brain components in MA abusers, compared to those of the controls.
3. Patients and Methods
3.1. Study Design
The current case-control study compared the stereological changes of the brain in MA abusers, compared to those of the healthy controls (n = 10 in each group). All of the participants were enrolled through the convenience sampling method among the MA abusers referred to the Baharan Hospital, Zahedan, Iran.
3.2. Participants
Twenty subjects were enrolled in the current study. The subjects in the case group were 10 individuals with MA addiction who referred to Baharan Hospital during the study. MA abuser participants were diagnosed and selected based on Diagnostic and Statistical Manual of Mental Disorders IV and text revision (DSM-IV TR) criteria for the study by an experienced psychiatrist. MA abusers enrolled in the current study had at least 6 months MA abuse experience and had not started any treatment and also abstinence from MA during this time. Ten subjects were selected as a control group, with no history of drug abuse as well as any psychological and neurological disorders based on physical examination by the same physician.
The study protocol was approved by the Ethics Committee of Zahedan University of Medical Sciences (ZAUMS) (Number: 90-2290). Written informed consent was obtained from all participants.
3.3. MRI Procedure and Stereological Estimation
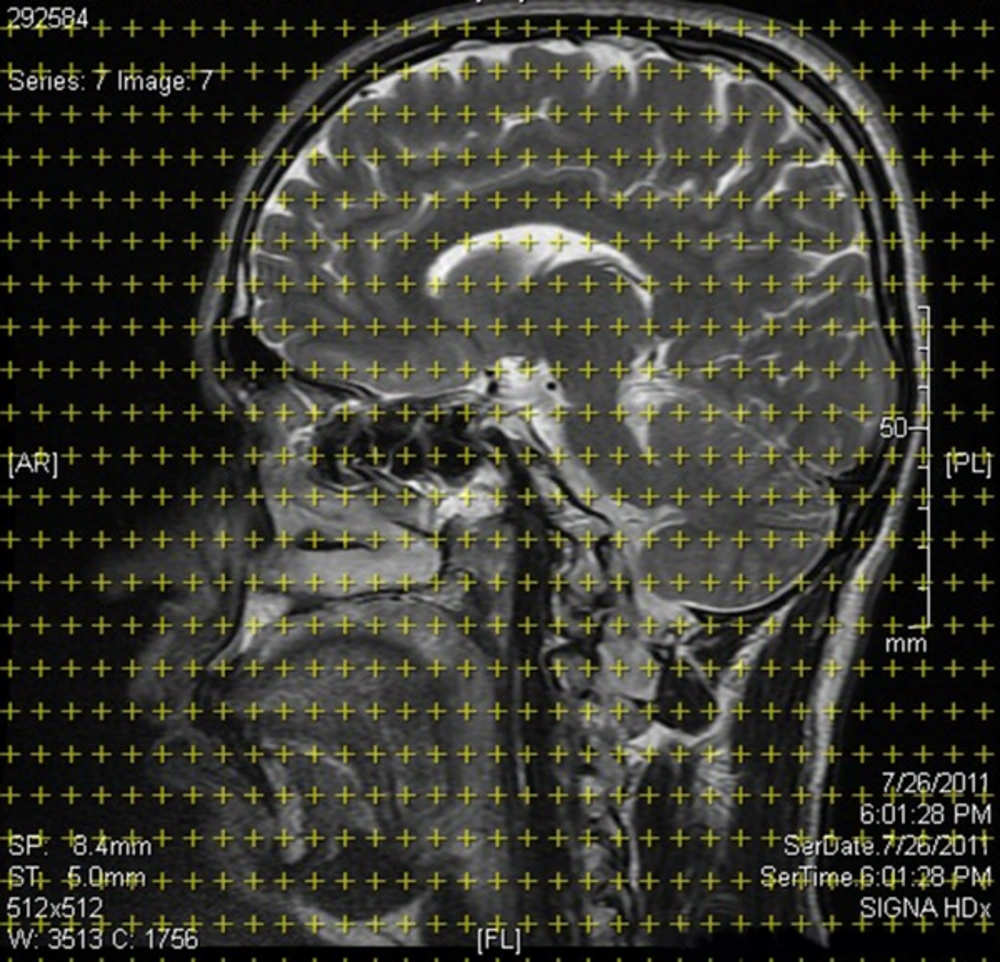
In order to estimate the stereological parameters of the brain in MA and healthy control groups, FLAIR (fluid attenuated inversion recovery) MR images were captured in frontal, coronal, and sagittal axes with 4-mm slide thickness and 0.5-mm interval at Ali-Ebne-Abitaleb hospital. The structural MRIs of the subjects were acquired by 3D high-resolution T1-weighted MRI 1/5 T scanner system (GE systems, Paris). On MR images with specified intervals, stereological grids contain organized points superimposed based on the Cavalieri point counting method, and the volumes and volume densities of desired brain regions were estimated and compared between the 2 groups (35, 36). All volumes of the desired regions of the brain were reported in cm3 (Figure 1).
The Cavalieri point counting method formula for volume estimation:

In the above equation, V is the estimation of the volume of any desired object, ∑P is the sum of the number of points hitting that object slices, a/p is the area associated with each point in the stereological grid (52.8 mm2), t is the mean distance between the captured slices, and M is the linear magnification of the image that was 13 in this case. Then, an estimate of the volume density (Vv) of the brain components in the reference space (ref: total brain) was obtained using:
Vv = P (part)/P (ref)
Where, P (part) is the number of test points falling in each component profiles and P (ref) is points hitting to the total brain (36-38).
3.4. Statistical Analysis
Data were expressed as mean ± standard error (SE) and to compare the stereological parameters among the 2 groups, the nonparametric Mann-Whitney U-test was used. All statistical analyses were performed with SPSS software for Windows (version 21, Chicago, IL, USA). Significance level was less than 0.05.
4. Results
All participants in the current study were male. The mean age of MA abuser and healthy controls were 27.40 ± 5.5 and 27.60 ± 6.31 years, respectively. There were no significant differences in terms of mean age between MA and the control subjects.
Results of the current study showed significant differences in cerebellum volume, ventricles volume and volume density (P = 0.011), gray matter volume and volume density, white matter volume and volume density between the 2 groups. On the other hand, there were no significant differences in the total brain volume, hippocampus and basal ganglia volume, and volume densities between the 2 groups (Table 1).
| Stereological Indices | MA Group (N = 10) | Control Group (N = 10) | Difference Percentage | P Value |
|---|---|---|---|---|
| Brain total volume, cm3 | 1054.7 ± 409.10 | 1151.1 ± 138.65 | -8.37 | NS |
| Hemispheres volume | ||||
| Total volume, cm3 | 916.5 ± 44.0 | 929.4 ± 126.15 | 1.38 | NS |
| Volume density, % | 87.0 ± 6.40 | 80.6 ± 2.50 | -7.94 | 0.023a |
| Left hemispheres volume | ||||
| Total volume, cm3 | 508.7 ± 38.41 | 509.3 ± 69.59 | 0.11 | NS |
| Volume density, % | 47.9 ± 4.38 | 44.0 ± 5.83 | -8.86 | NS |
| Right hemispheres volume | ||||
| Total volume, cm3 | 407.9 ± 65.13 | 420.0 ± 97.99 | 2.88 | NS |
| Volume density, % | 38.3 ± 7.01 | 35.7 ± 5.60 | -7.28 | NS |
| Cerebellum volume | ||||
| Total volume, cm3 | 125.4 ± 10.27 | 139.5 ± 20.58 | 10.10 | 0.035a |
| Volume density, % | 11.5 ± 1.08 | 11.7 ± 1.57 | 1.70 | NS |
| Ventricles volume | ||||
| Total volume, cm3 | 43.0 ± 6.20 | 34.5 ± 8.86 | -91.97 | 0.029a |
| Volume density, % | 3.6 ± 0.70 | 2.5 ± 0.84 | -44 | 0.011a |
| Left ventricles volume | ||||
| Total volume, cm3 | 20.3 ± 2.29 | 16.6 ± 4.52 | -22.28 | NS |
| Volume density, % | 1.6 ± 0.52 | 1.5 ± 0.43 | -6.66 | NS |
| Left ventricle/hemisphere, % | 3.9 ± 0.37 | 3.4 ± 1. 27 | -14.70 | NS |
| Right ventricles volume | ||||
| Total volume, cm3 | 22.7 ± 5.78 | 18.4± 5.11 | -23.36 | NS |
| Volume density, % | 1.7 ± 0.49 | 1.6 ± 0.36 | 53.75 | NS |
| Right ventricle/ hemisphere, % | 5.6 ± 1.71 | 4.5 ± 1.74 | -24.44 | NS |
| Hippocampus volume | ||||
| Total volume, cm3 | 9.2 ± 1.09 | 11.5 ± 2.94 | 20 | NS |
| Volume density, % | 0.94 ± 0.11 | 1.0 ± 0.01 | 6 | NS |
| Gray matter volume | ||||
| Total volume, cm3 | 377.5 ± 40.73 | 530.9 ± 187.50 | 28.89 | 0.03a |
| Volume density, % | 35.7 ± 2.54 | 45.1 ± 1.10 | 20.84 | 0.043a |
| White matter volume | ||||
| Total volume, cm3 | 677.3 ± 10.71 | 620.2 ± 75.07 | -9.20 | 0.015a |
| Volume density, % | 64.2 ± 2.54 | 54.9 ± 10.95 | -16.93 | 0.043a |
| White/gray matter ratio | 1.8 ± 0.20 | 1.3 ± 0.54 | -38.46 | 0.043a |
| Basal ganglia volume | ||||
| Total volume, cm3 | 14.4 ± 1.65 | 11.5 ± 3.98 | -25.21 | NS |
| Volume density, % | 1.3 ± 0.16 | 1.0 ± 0.29 | -30 | 0.009a |
Abbreviation: NS, not significant.
aSignificant level P < 0.05.
5. Discussion
The results of the current study showed significant differences in volumes of the brain structures in between the 2 groups.
Structural MR imaging studies revealed that a long-term substance abuse can cause enlargement and atrophy in various regions of the human brain. These results, as starting points for further researches, may lead to discovering the mechanisms of enhancing the volumetric changes and their implications on physical, psychological and cognitive changes following the substance abuse (39).
According to the current study, cerebellum, ventricles and gray matter volume and volume densities in MA abusers were significantly lower than those of the control group. White matter volume and volume density in MA abusers were larger than those of the control group. Morales et al., using voxel-based morphometric (VBM) technique showed reduction in cerebellum volume in MA abusers (40). Another study also showed that MA chronic abuse in low-dose (LD) and high-dose (HD) could cause reduction of the molecular and granular layers of the cerebellum. Reduction of the volume in HD group was significantly greater than that of the LD group (33). It seems that MA in a dose-dependent manner can cause induction of apoptosis and cell death and reduce in the cerebellum volume after a long-term MA abuse (23, 33, 41).
Findings of the current study also revealed that the white matter volume in MA addicts was significantly larger than the control group. The current study results were consistent with those of Thompson et al. (31) and Ardakani (33). They proposed that MA long-term abuse could cause increment of the white matter volume. This process is probably done as a compensatory process in myelin producing cells to offset the deficits occurred in gray matter of the brain following the MA abuse. Thus, it can be concluded that increment of white matter volume in MA abusers compensate for the reduction of gray matter; on the other hand, reduction of white matter volume was reported by Aoki in patients with schizophrenia following the gray matter reduction (42). The contradiction can be attributed to different natures of the disorders. The researchers speculated that the reason for white matter hypertrophy followed by MA abuse can be mutate myelination, adaptive glial cell proliferation, and neuropil reduction (31, 43).
Thompson et al., showed that hippocampal volume in MA abusers was significantly smaller than that of the controls. They found sever atrophy in the hippocampi of MA abusers, compared to the controls. These volume deficits were accompanied by reduction of memory function in MA abusers. They also found a positive correlation between right and left hippocampus volumes and the individuals with bilateral hippocampal atrophy had a poorer performance on a word-recall task. Despite the volumetric changes in hippocampus, there were no significant differences in the total cerebral volume between the 2 groups (31). Thanos et al. showed no significant differences in the hippocampus, findings in this sense were consistent with those of the current study results. It seems that contradiction is some studies can be due to differences in time-duration and dose of MA abused by their samples (44, 45). Mathias, in an experimental study on mice, reported volume reduction in hippocampus. He stated that this volume reduction was due to hippocampal cell death following the induction of apoptosis by MA (41). Another study also reported that MA use can cause cell death through the DNA damage and apoptosis process (21). Overall, it can be suggested that volume reduction in various regions of the brain, particularly in hippocampus, following the chronic MA abuse could be due to activation of cell death mechanisms and induction of apoptosis.
Basal ganglia is a mass of gray matter, surrounded by white matter, comprised of caudate nucleus, putamen and globus pallidus (lentiform nucleus), substantia nigra pars compacta (SNpc), and subthalamic nucleus. These nuclei communicate with each other and with motor centers to control the motor system activities. Therefore, any damages in components of this system can cause movement disorders in human beings (46). A study conducted by Thanos et al. on the effects of chronic MA addiction on brain structure and function showed an increment in the striatal volume in long-term MA treatment. They stated that this increment was uniform in the whole of the striatum. Other studies in this field also showed that the striatum volume increased following the chronic MA abuse. Even this striatum volume increment occurred in both MA active abusers and abstinence (44, 45, 47). Moreover, Ares-Santos et al., showed that MA abuse caused long-lasting loss/degeneration of dopaminergic cell bodies in the SNpc, along with destruction of dopaminergic terminals in the striatum (48). It seems that striatal enlargement can be one of the major findings in MA long-term abuse. Corpus striatum is an important part of the basal ganglia that plays an important role in facilitating and calm movements (46). According to role of the striatum in dopamine (DA) transmission pathways, any damages and changes in it can lead to the motor disorders (3, 49). Probably defects in these pathways and imbalance in DA levels in the brain can be the reason for the motor disorders in MA abusers (50, 51).
5.1. Conclusions
Accordingly, it seems that stereological technique can be used to assess vast and various parameters such as size, volume, and length in neurodegenerative disorders in the brain structures. Quantitative data obtained from stereology are accurate indicators to judge structural abnormalities and route of their treatments.