1. Background
Jaundice is a common symptom in the newborn; it is detected in 60% of term newborns and 80% of preterm newborns (1). It can reach levels that require phototherapy in about 10% of term newborns and 25% of preterm newborns (2). Although it is generally a temporary and benign condition, it can cause neurologic sequelae through bilirubin encephalopathy and irreversible severe brain damage in a small portion of the newborns (3).
Total serum bilirubin (TSB) levels are a guide for diagnosis, therapy, and follow-up. To determine TSB, blood samples from the newborn and laboratory tests are required. Venous or capillary blood sampling from newborns is a painful process. Moreover, repeat drawing of blood poses a risk for anemia and infection (4). Fast and easy TSB level detection with a non-invasive method is possible with transcutaneous bilirubin (TcB) measuring (Bilicheck) devices (5). In the literature, a high correlation between TcB and TSB measurements in term and preterm babies has been emphasized (6-8). The easy use and non-invasive TcB measurement facilitated the management of newborn jaundice and reduced jaundice complications (3, 9-11).
Serum bilirubin levels are increased after phototherapy treatment. This is also known as increase of rebound bilirubin, which is caused by bilirubin generation and clearance changing. Blood TSB levels are checked for rebound bilirubin values. Transcutaneous bilirubin (TcB) measuring may give incorrect results if performed during the phototherapy period or immediately after phototherapy treatment.
2. Objectives
There are limited data about the proper TcB measuring time to detect levels that are close to serum bilirubin levels after ending the phototherapy treatment. The aim of this study was to compare the TcB and TSB levels after the phototherapy treatment of newborns, and to test the reliability of this treatment method.
3. Methods
3.1. Study Design
This study was performed in the newborn units of Tekirdağ Namık Kemal University, between July-2018 and May-2020. The Local Ethics Committee approved the current study (No.: 2020.93 .04.17). Late preterm (n: 10) and term babies (n: 95) aged between 350/7 and 416/7 were involved in the study.
Gestational age was calculated according to the last menstrual period. Babies who had no complication other than hyperbilirubinemia were included in the assessment. Deliveries with intrapartum and postpartum complications, ABO and/or Rh hemolytic disease, cyanotic babies, extended jaundice, neonatal sepsis, direct hyperbilirubinemia, skin disease, and babies with a history of previous phototherapy or exchange transfusion were excluded from the study.
A Bilicheck (M&B-MBJ20) transcutaneous bilirubin device (bilirubinometer) was used for the transcutaneous bilirubin measurements. The device was calibrated according to the manufacturer's instructions. In patients, the device was applied to the midsternal areas with slight pressure and measured three times. Then, TcB values were obtained as the average of these three measurements.
For the phototherapy treatment, nomogram of phototherapy limits and gestational week, postnatal age, total bilirubin level (mg/dL) and additional risk factors were considered according to the American Pediatric Academia and Turkish Neonatology Committee, Newborn Jaundice Guide (3). Each baby received 10 hours of single phototherapy treatment. After the end of phototherapy, TcB and TSB were measured at 6th and 12th hours.
To compare the TcB and TSB values, simultaneous measurements were performed immediately after or before taking blood within a maximum ± 15 minutes. The venous blood was placed in additive-free gel BD vacutainer (SSTTM II Advance, UK) biochemistry tubes and then transferred to the biochemical laboratory. TSB levels were studied using spectrophotometry with an autoanalyzer device.
3.2. Data Analysis
Data were analyzed using the SPSS for Windows ver 22.0 device (IBM Corp, New York, USA). The Shapiro-Wilk test was used to determine the normality of distribution of TSB and TcB data. To analyze the correlation between simultaneous TcB and TSB measurements, Pearson’s correlation test and linear regression analyses were used.
4. Results
The demographic features of the patients are shown in Tables 1 and 2.
| Mean ± SD | Max-min | |
|---|---|---|
| Birth Weight (g) | 3250 ± 420 | 3900 - 2650 |
| Gestation age (weeks) | 373/7 ± 1.9 | 416/7 - 352/7 |
| Mother's age (years) | 29 ± 6 | 17 - 43 |
| Phototherapy time (hour) | 10 ± 2 | 12 - 8 |
| No. (%) | |
|---|---|
| Sex | |
| Female | 56 (53.3) |
| Male | 49 (46.6) |
| Postnatal age (hour) | |
| 24 - 48 | 43 (40.9) |
| 49 - 72 | 28 (26.6) |
| 73 - 96 | 14 (13.3) |
| 97 - 120 | 12 (11.4) |
| > 120 | 8 (7.6) |
| 24 - 48 | 43 (40.9) |
The 6th hour TcB levels, which were measured on the sternum, ranged between 5.8 and 12.4 mg/dL; the average was 8.6 ± 2.5 mg/dL, and the median was 8.7 mg/dL). The simultaneously measured 6th hour TSB levels were in average 11.2 ± 2.6 mg/dL, and the median was 11.3 mg/dL (Table 3). The 12th hour TcB levels, which were measured on the sternum, ranged between 6.1 and 12 mg/dL, the average was 9 ± 2.4 mg/dL, and the median was 9 mg/dL. The simultaneously measured 12th hour TSB levels were 6.4 - 12.6 mg/dL, the average was 9.5 ± 2.6 mg/dL, and the median was 12.4 mg/dL. The hematocrit levels of the patients was 40 - 66%, the average was 53 ± 4.8%, and the median was 51%. The highest total bilirubin was 18 mg/dL.
| Mean ± SD | Median | Min-Max | |
|---|---|---|---|
| TcB 6th hour (mg/dL) | 8.6 ± 2.5 | 8.7 | 5.8 - 12.4 |
| TSB 6th hour (mg/dL) | 11.2 ± 2.6 | 11.3 | 7.5 - 14.5 |
| TcB 12th hour (mg/dL) | 9 ± 2.4 | 9 | 6.1 - 12 |
| TSB 12th hour (mg/dL) | 9.5 ± 2.6 | 12.4 | 6.4- 12.6 |
Abbreviations: TcB, Transcutaneous bilirubin; TSB, Total serum bilirubin.
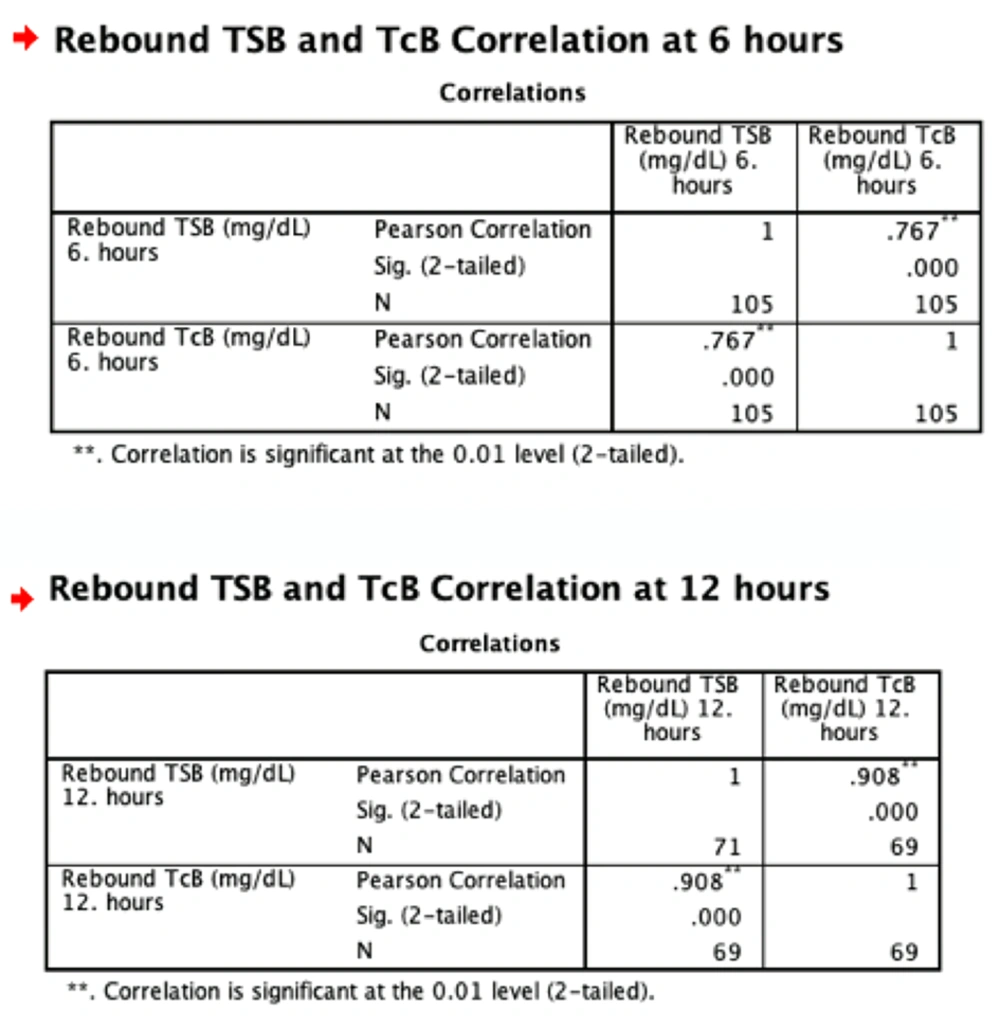
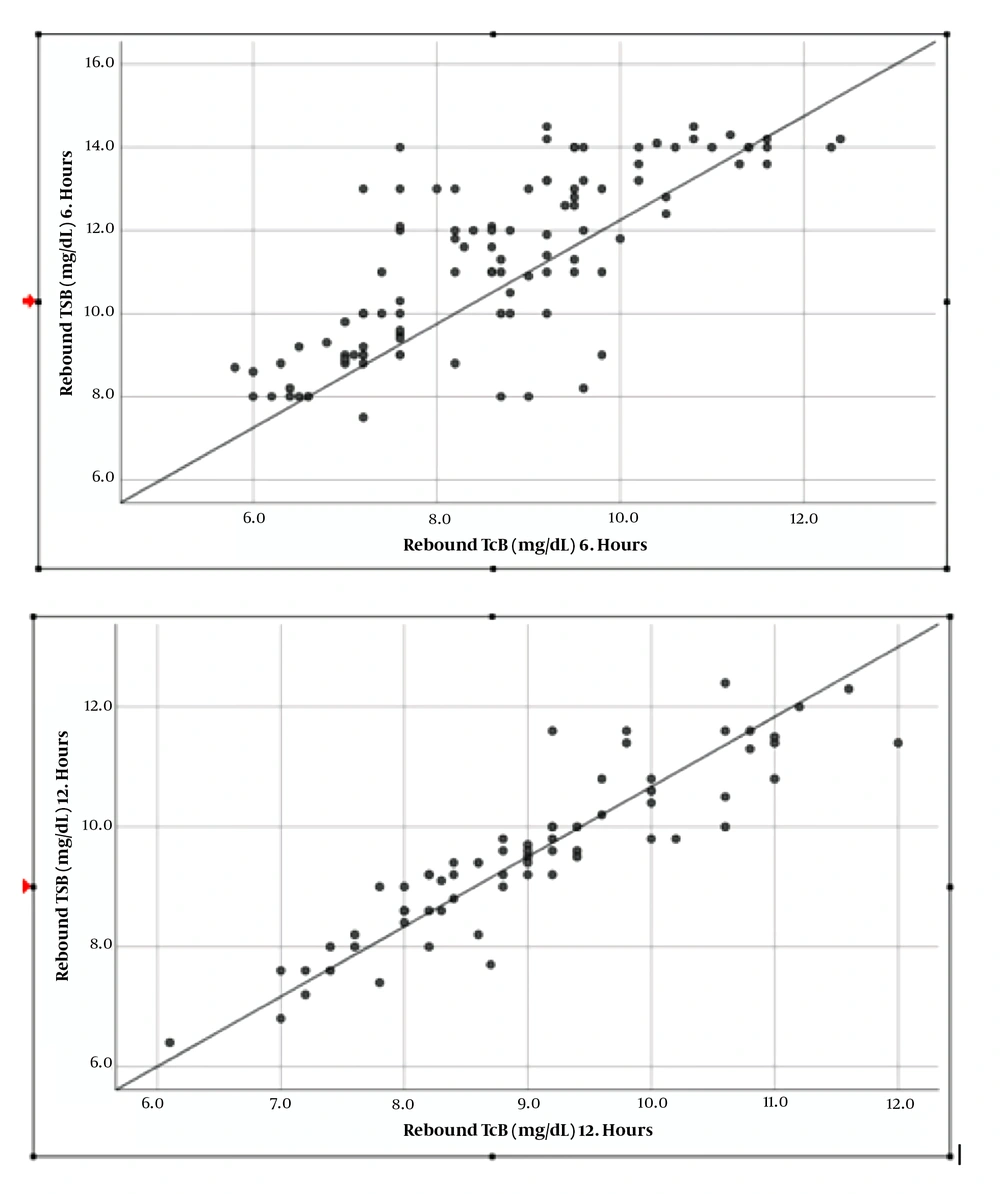
After phototherapy treatment, the correlation between TcB and TSB was high (r = 0.76) at the 6th hour, and was very highly correlated (r = 0.90) at the 12th hour (Figures 1 and 2, Table 4).
| r | P Value | |
|---|---|---|
| 6th hour | 0.767 | < 0.01 |
| 12th hour | 0.908 | < 0.01 |
Abbreviations: TcB, Transcutaneous bilirubin; TSB, Total serum bilirubin.
ar, pearson correlation coefficient **P < 0.01
5. Discussion
In our study, we detected that the correlation was much greater at 12 hours when we compared the TcB and TSB measurements after phototherapy treatment received by newborns with hyperbilirubinemia aged ≥ 35 gestational weeks.
TcB levels measured on the forehead and sternum were found similar in other studies (12, 13). Our study results showed that 6th and 12th hour rebound bilirubin values indicated a correlation between TcB and TSB in preterm and term newborns after phototherapy treatment. Juster-Reicher et al (14) evaluated simultaneously measured TcB and TSB from 171 newborns and found correlation coefficients as 0.56, 0.73, 0.65, 0.71, 0.72, 0.68, and 0.80 at 1 - 8 hours, 9 - 16 hours, 17 - 24 hours, 25 - 48 hours, 49 - 72 hours, 79 - 95 hours, and > 96 hours, respectively.
Grabenhenrich et al (4) evaluated the correlation between TcB and TSB in 86 newborns for the first 8 hours, 8-16 hours, 17 - 24 hours and > 24 hours, and they found that the lowest correlation occurred within 1 - 8 hours. Akın et al (15) suggested that transcutaneous bilirubin might be measured at 7 hours as the earliest after phototherapy. Jnah et al (16) showed that the TSB and TcB level correlation was high at 24 hours after phototherapy (r = 0.869).
In the present study, the correlation between TcB and TSB was highly correlated at 6th hour (r = 0.76) and very highly correlated at 12th hour after phototherapy treatment (r = 0.90).
The low correlation within 8 hours in previous studies can be explained by measuring the serum and transcutaneous bilirubin levels within 8 hours or measuring the TcB levels in early stages with lower reliability. Moreover, we suggest that transcutaneous bilirubin measuring devices may give different results because of different populations with different skin colors (17, 18). In our study, serum and transcutaneous bilirubin values were measured only at the 6th and 12th hours (max ± 15 minutes).
The limitation of the study was using only one type and one brand of transcutaneous device, and measurements were performed only on the sternum. The melanin content of the skin changes within a couple of weeks after delivery and thus transcutaneous evaluations can be affected.
5.1. Conclusion
In our study, TcB measurement in jaundice screening on the skin was quite reliable within 6-12 hours after phototherapy. This might help to prevent repeated blood collections from the heel. However, it is necessary to reevaluate the results by studies conducted with larger sample size.