1. Background
The ductus arteriosus connecting pulmonary artery to aorta, is an essential structure during fetal life. Because fetal pulmonary vascular resistance is high, nearly 90% of the blood ejected by the fetal right ventricle flows through ductus arteriosus to descending aorta. The ductus arteriosus generally closes during the first few days after birth, and entire right ventricular output is ejected into the pulmonary arterial bed. If ductus arteriosus remains patent postnatally and pulmonary vascular resistance falls, then the blood flow through ductus arteriosus reverses from aorta to pulmonary artery. Because this connection is open during systole and diastole and the pulmonary artery pressure is lower than the aortic pressure, blood flows through the patent ductus arteriosus throughout the cardiac cycle. This volume overload results in enlargement of the pulmonary artery, pulmonary veins, left atrium and left ventricle not only by volume of blood through the PDA and its sizes, but also because of the relative differences in the systemic and pulmonary vascular resistances (1).
Indomethacin and Ibuprofen are the drugs of choice to close PDA with success rate of 70% - 80% except for the conditions such as renal failure, oliguria, distinct thrombocytopenia, active bleeding, necrotizing enterocolitis (NEC) and bowel perforation (2). Failing the drugs or presence of any contraindication, patients would be candidates for surgical closure. Once paracetamol is widely used as a pain controller for neonates, many experiments are ensuing from. Thus it attracts attentions if be otherwise effective for PDA closure with less adverse effects (3, 4).
Prevention of ductus arteriosus patency, by a less adverse and more suitable drug attenuates mortality, morbidity, neurodevelopmental complications and also hospital stay that results in decreased expenditure burdened on patient’s family. Previous studies have elucidated that intravenricular hemorrhage and especially that of grades 3 and 4 are reduced by indomethacin prophylaxis started whithin 12 hours of birth but did not correlate with neurodevelopmental outcomes (1). Due to lack of a trial looking up to prophylactic role of parenteral paracetamol on PDA closure in Iran, we were made to perform it with a larger number of neonates and wider range of gestational age.
2. Methods
This is a randomized clinical trial conducted on the neonates admitted to the NICU of Afzalipour tertiary hospital, Kerman, Iran, between November 2015 and 2016. The neonates with gestational age of ≤ 34 weeks were recruited and the other demographic characteristics such as age, gender and severity of respiratory distress syndrome (RDS) in both groups were matched. Those with pulmonary artery atresia, aortic coarctation, genetic disorders, persistent pulmonary hypertension (PPHN), severe asphyxia, hepatic failure, 5th-minute Apgar score < 5 and cord blood pH < 7.00, were excluded. As routine, meticulous physical examination such as heart and lung auscultation seeking murmurs and four-limb pulse oxymetry and four-limb blood pressure measurement were performed shortly after birth to predict any baby with likely ductus-dependent cardiac anomaly because in this case initiation of paracetamol and ensuing ductus closure could be catastrophic. Totally 160 neonates were included equally divided into case and control arms. The case neonates were given prophylactic parenteral paracetamol (20 mg/kg stat and 7.5 mg/kg every 6 hours) during first three days of life whereas control group received no drugs. Of course, the first dose of paracetamol was injected after 12 hours of birth that it gave a good opportunity to examine the neonates carefully for cyanosis or pathologic murmurs and monitor them by four-limb pulse oxymetry and blood pressure as said before in which any suspicion or evidence of congenital heart diseases or genetic disorders led to exclusion of the baby and prompt emergent echocardiography and other needed interventions as well. The applied paracetamol vial contained 1 gr/6.7 mL (Tsetis Pharmaceuticals.Uni.Pharma Kleon, Greece, Serial No. Pharma-Sa-Lot-1591, Classification No. N02BE01). Balanced block randomization was used to remove nuisance factors. All parents of eligible neonates were spoken about beneficial and adverse effects of paracetamol, PDA ventures and other details of the study. Those whose parents filled consent form were included. The trial obtained the Iranian ethical codes IR.KMU.REC.1395.841 and ID: IRCT2017012718994N2.
All 160 neonates underwent echocardiography after passing the gap of first 3 postnatal days to detect any PDA if yet remained open to promptly start Ibuprofen treatment. The hemodynamically unstable neonates and those who deteriorated or showed impaired liver function tests during the gap; needed paracetamol cessation and start of Ibuprofen administration and henceforth they were excluded. The liver function tests (LFTs) included the liver enzymes assay (AST, ALT, AlkP), PT (prothrombin time) and total serum protein, which were daily checked during first 3 days of birth (totally 3 times). The primary LFTs were obtained from all neonates of the case group prior to initiation of paracetamol to be sure of hepatic insufficiency.
Echocardiography (Samsung, N. Korea, 5.0 MHz Probe).was performed by two pediatric cardiologists who were absolutely unaware of case and control groups and neonate conditions. In addition, the nurses in charge of paracetamol injection were unaware of case-control division and the reason of the drug injection because sometimes paracetamol was used as analgesic in our ward.
Data were analyzed by SPS software ver. 24. Quantitative and qualitative data were described by mean and standard deviation respectively. Chi-square test and t-test were used to compare qualitative and quantitative variables respectively at the significance of 0.05. Balanced block randomization was used to remove confounding factors.
3. Results
Out of 160 neonates, 87 (54%) were male. Mean weights of case and control groups were 1400.31 ± 217.77 gr and 1355.25 ± 245.92 gr respectively. There was no significant difference between the groups (P = 0.11). Means of gestational age were 30.36 ± 1.19 weeks and 30.38 ± 1.44 weeks in case and control groups respectively. No significant difference was found (P = 0.48). Mean time of hospitalization for control group was more than that for case group but the difference was not significant (20.45 ± 9.25 days vs. 18.35 ± 8.90 days) (P = 0.06)
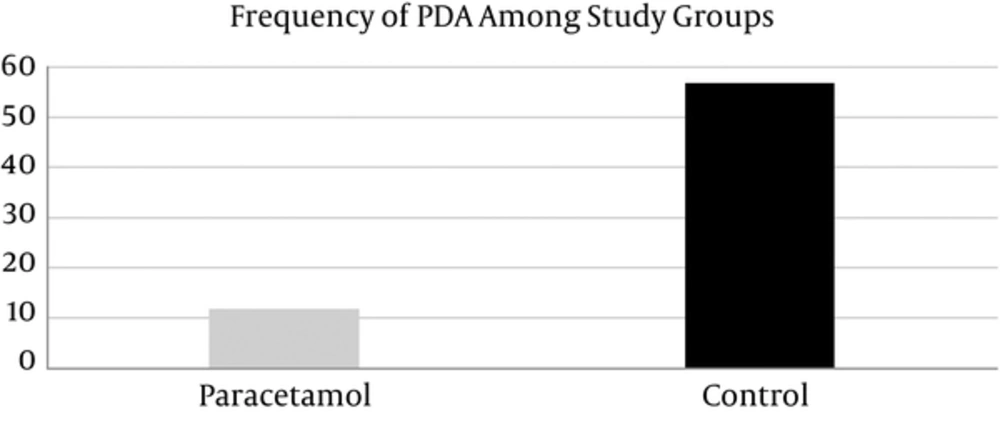
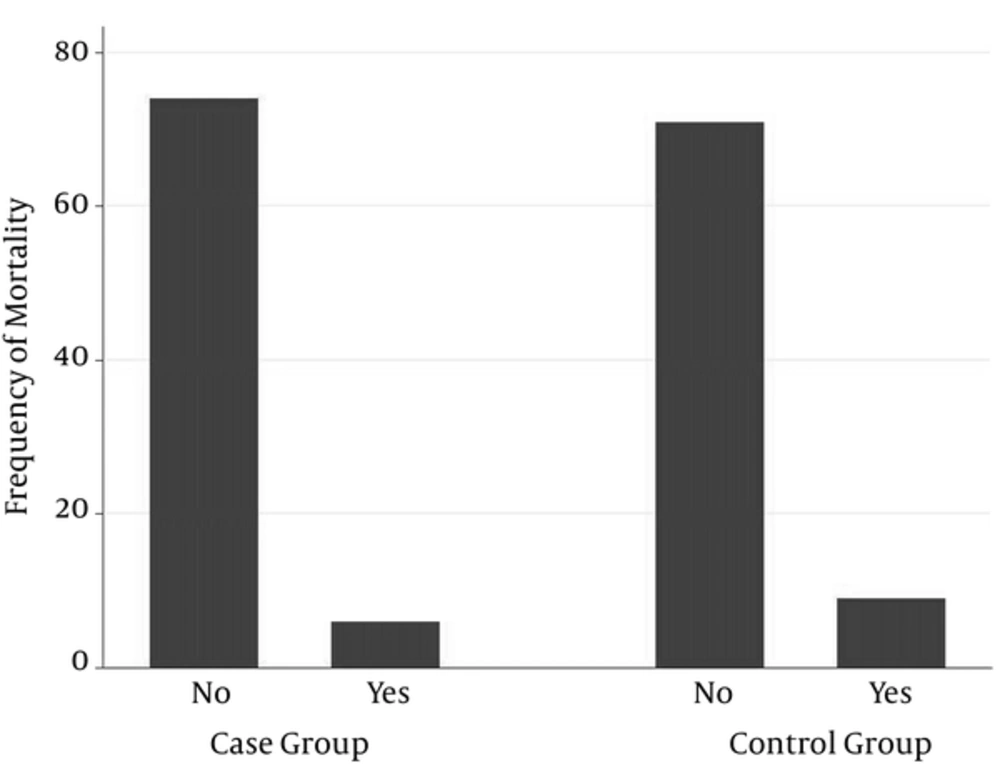
PDA frequency in case group was less than that in control group (12 (15%) vs. 57 (71.25%)). This difference was statistically significant (P < 0.001) (Table 1, Figure 1) whereas none of the following differences was significant, Mean ventilator time needed was 1.66 ± 3.22 days in case group and 2.28 ± 3.85 days in control group (P = 0.14) (Table 1). Means cardiac fraction shortened was 51.39 ± 4.10 in case group vs. 51.27 ± 4.81 in control group. (P = 0.43) (Table 1). 6 (7.5%) neonates died in case and 9 (11.25%) in control group. (P = 0.42) (Table 1, Figure 2). Atrial septal defect (ASD) frequencies of case and control groups were 18 (22.5%) and 25 (31.25%) respectively. (P = 0.21). Ventricular septal defect (VSD) was absent in case group and only one neonate in control group had it. (P = 0.32). Tricuspid valve regurgitation (TR) frequencies in case and control groups were 10 (12.5%) and 14 (17.5%) respectively. (P = 0.38). Mitral valve regurgitation (MR) frequencies in case and control groups were 5 (6.25%) and 3 (5%) respectively (P = 0.47). In addition, no hepatic complications occurred during hospitalization of case neonates.
| Variable | Group | P Value | |
|---|---|---|---|
| Case | Control | ||
| PDA frequency | 12 | 57 | < 0.001 |
| Shortening fraction | 51.39 ± 4.10 | 51.27 ± 4.81 | 0.43 |
| Mortality rate | 6 | 9 | 0.42 |
| Days of need to ventilator | 3.22 ± 1.66 | 3.85 ± 2.28 | 0.14 |
| Days of Hospitalization | 18.35 ± 8.90 | 20.45 ± 9.25 | 0.06 |
4. Discussion
At the present time, Ibuprofen and Indomethacin are drugs of choice for PDA treatment and prophylaxis. Trials on paracetamol efficacy are still lacking and limited to when the conventional drugs fail to or complicate closing ductus arteriosus; for example, Gournay and colleagues compared prophylactic effects of Indomethacin and Ibuprofen. Both showed success in preventing ductus arteriosus patency, but led to renal failure and pulmonary hypertension that coerced the executors to abandon the study and paracetamol was consequently considered as alternative (5). Hammerman and associates reported 5 neonates with 26 - 32 weeks of gestational age and 3 - 35 post-natal days who were given oral paracetamol (15 mg/kg every 6 hours for 2 days) because of Ibuprofen contraindication or incapability for PDA closure. Surprisingly, paracetamol showed successful effect in all of them (6).
Terrin and co-workers (2014) also performed a trial in which 8 pretem neonates coercively received paracetamol owing to contraindications of the conventional drugs. In 6 (75%) of them PDA resolved with no adverse effects (7). Conversely, Alan and associates conducted a similar study in which neither Ibuprofen nor paracetamol showed capable to close patent ductus arteriosus (8).
Overall, two investigator teams have studied prophylactic paracetamol; firstly Aikio and co-workers(2008 - 2011) applied the prophylactic dose of parenteral paracetamol (20 mg/kg stat and 7.5 mg/kg every 6 hours for 3 postnatal days), recruiting 109 very preterm neonates with gestational age of ≤ 32 weeks as the case group. Consequently, the PDA incidences were 14.7% in case and 30.7% in control groups. paracetamol was unequivocally associated with reduction of PDA incidence (P = 0.008). In 15 neonates of case and 26 of control groups ductus arteriosus closure failed; thus, Ibuprofen needed to be used. Albeit, 3 neonates of case and 7 of control groups were enlisted for surgery. The “days of need to ventilator” did not associate with paracetamol (P = 0.68) (3). Secondly, Akbari Asbagh and colleagues (2011) studied 32 very preterm neonates who received oral prophylactic paracetamol (15 mg/kg every 6 hours for 2 postnatal days). Echocardiography at 4th postnatal day revealed open ductus arteriosus in 4 neonates of case and 8 of control groups. Oral paracetamol was not associated with reduction of ductus arteriosus patency (P = 0.273) (9).
Similar to Aikio’s trial, we studied totally 160 newborn infants who were equally divided into case and control groups. Parenteral prophylactic paracetamol (20 mg/kg stat and 7.5 mg/kg every 6 hours for 3 postnatal days) was used for the case group. Henceforth, at-the-day-4 echocardiography was done for all of 160 neonates. The detected PDA cases in case group were 12 (15%) and in control group 57 (71.25%) (P < 0.01). So, parenteral prophylactic paracetamol was associated with reduction of PDA incidence.
The rest of variables e.g. days of need for ventilator, days of hospitalization and mortality rate did not associate with paracetamol which could be resulted from the various confounding factors including maternal factors (diabetes mellitus, premature rupture of membranes, prenatal infections), ambient factors (hypothermia) and neonatal factors (pulmonary hemorrhage, intraventricular hemorrage).