1. Background
Nephrotic syndrome (NS) is a clinical condition characterized by massive proteinuria, hypoalbuminemia, hypercholesterolemia, and generalized edema (1).
Children with NS may be at risk for metabolic bone disease because of biochemical derangement caused by renal disease as well as steroid therapy (2).
Previous studies show that some markers of bone mineral density (BMD) were affected during steroid therapy (after beginning and within three months of therapy). These markers include mean serum calcium which is found to be significantly lower than those at the beginning of therapy. Mean serum alkaline phosphatase and urine calcium/ creatinine ratio (Ca/Cr) are significantly increased in comparison to the beginning of therapy (3).
Osteocalcin (OC) is a non-collagenous, 49 amino acid glutamate-rich polypeptide bone matrix with a molecular weight of about 5800 kDa (4).
OC functions as an inhibitor of bone mineralization. It inhibits the precipitation of calcium salts from saturated solutions (5). The serum concentrations of bone-specific alkaline phosphatase and OC reflect cellular activity of osteoblasts (6).
OC level is related to increased bone turnover and it decreases in patients taking anti-resorptive agents (biphosphonates or hormone replacement therapy (HRT)); this usually occurs within 3 - 6 months after therapy begins. Decreasing levels indicate effective response to treatment (7).
The aim of this study was to evaluate linear growth and bone turnover markers (calcium, phosphorous, alkaline phosphatase and serum osteocalcin) in children with steroid-dependant/ frequently-relapsing (SRNS/FRNS) and steroid resistant nephrotic syndrome (SRNS) in comparison to a matched control group. It aimed also to assess whether there is a significant affection on growth regarding height and weight of these patients under long-term steroid therapy in comparison to the control group.
2. Methods
Our study is a cross-sectional study conducted at University children’s hospital, faculty of Medicine, Cairo University, Egypt from July 2014 to August 2015. It included 60 patients, aged 2 - 15 years recruited from outpatient nephrology clinic. Duration of the disease had to be not less than six months. Informed consent was taken from each parent to participate in the study.
Twenty eight age- and sex-matched healthy children were included as a control group. They were recruited from the follow-up and surgical clinics of the hospital after consent from their parents. They had no history of chronic illness or any received medications known to affect bone turnover.
Patients with renal impairment or renal tubular defect, patients with steroid-sensitive nephrotic syndrome, patients with congenital nephrotic syndrome, patients with duration of the disease less than 6 months and with other causes of low bone density (vitamin D deficiency, metabolic disorders such as irregularities in thyroid and parathyroid hormones and medications such as anticonvulsants, etc.) were excluded from the study.
Patients were divided into 3 groups; Group A included 30 children with SDNS/FRNS, Group B included 30 children with SRNS and Group C included 28 matched children of the same age and sex as the control group. SRNS is defined as failure to develop remission after six weeks of prednisone at dose of 60 mg/m2/day, SDNS is defined as acquiring two consequent relapses during corticosteroid withdrawal or 14 days after treatment was completed and FRNS is defined as acquiring of two or more relapses within 6 months of initial relapse or four or more relapses after 10 months of remission (8).
The sample size provided a power of 80% assuming a mean control OC level of 20 ng/mL and intergroup variation of at least 25%
Patients were subjected to the following;
- Full history taking, including age of onset, duration of illness, number of relapses, presence of hypertension and hematuria, symptoms suggestive of osteoporosis (e.g. back pain, spinal deformity, recurrent long bone fractures, etc.), history of calcium and vitamin D supplementation during course of the disease and detailed information about the modalities of therapy and pattern of steroid response.
- Clinical examination, including; height and weight for age percentiles according to Egyptian growth curves to assess the growth status of the children, assessment of blood pressure and presence of oedema.
- Laboratory investigations, including; serum urea and creatinine, serum albumin, cholesterol, serum calcium, phosphorus and serum alkaline phosphatase (analyzed by Dimension auto-analyzer). Protein/creatinine ratio in urine was done using Beckman Synchron CX9 (Beckman Coulter). Serum osteocalcin was measured by immune-radiometric assay (IRMA).
2.1. Statistical Analysis
All data were collected on standardized forms, entered in a computerized database, and analyzed with statistical software packing using Microsoft Excel 2013 and SPSS version 22.0.
Results were statistically presented in terms of (Range, mean, median, standard deviation and percentage). Continuous data (of both groups) were compared with paired T-tests and categorical data (parametrical data) by Pearson’s chi-square test was performed. A P value < 0.05 was considered statistically significant (9).
3. Results
The age of the SDNS/FRNS group was 8.5 ± 3 years (range: 2 - 15), the age of the SRNS group was 9.4 ± 3 years (range: 2 - 15) while of the control group was 7 ± 3 years (range: 3 - 14) with no statistically significant differences between the groups. Twenty (66.7%) patients of the SDNS/FRNS group were males and 10 (33.3%) females, 19 (63.3%) patients of the SRNS group were males and 11 (36.7%) females, while 13 (46.4%) of the control group were males and 15 (53.6%) females.
Age of onset was 3.6 ± 2.4 years in SDNS/FRNS group and 4.4 ± 2.6 in SRNS patients. Number of relapses during the whole course of the disease was 4 (range; 2 - 14) in both SDNS/FRNS and SRNS groups. Twenty (66.7%) patients of the SDNS/FRNS group and 16 (53.3%) patients of the SRNS group were in remission at the time of the study.
Table 1 shows anthropometric measures of each of the study groups separately in comparison to control group. Regarding height of our patients, both of our patient groups (SDNS/FRNS and SRNS) showed lower height for age percentile compared to control group (P = 0.017 and 0.001, respectively). In addition, we found that 50% of our patients were below 5th percentile for height.
| Variables | Study Group | Control Group | P Value |
|---|---|---|---|
| Weight for age percentile | 51.8 ± 21.7 | ||
| SDNS/FRNS | 70.42 ± 26.7 | 0.001** | |
| SRNS | 63.7 ± 28.6 | 0.082 | |
| Height for age percentile | 35.7 ± 25.1 | ||
| SDNS/FRNS | 20.2 ± 22.7 | 0.017* | |
| SRNS | 9.9 ± 18 | 0.001** | |
| BMI | 17.3 ± 2.9 | ||
| SDNS/FRNS | 22.3 ± 4.4 | 0.001** | |
| SRNS | 23.8 ± 6.8 | 0.001** |
Abbreviations: BMI, Body Mass Index; SRNS, Steroid-Resistant Nephrotic Syndrome; SDNS/FRNS, Steroid-Dependent / Frequent-Relapsing Nerphrotic Syndrome.
Table 2 shows number of patients who had bone affections before and after starting of steroid therapy and number of patients receiving prophylactic calcium and vitamin D during childhood period.
| Variables | SDNS/FRNS | SRNS | Total |
|---|---|---|---|
| No bone affection before steroid treatment | 30 (100) | 30 (100) | 60 (100) |
| Bone affection after steroid treatment | |||
| No | 25 (83.3) | 25 (83.3) | 50 (83.8) |
| Bone aches | 4 (13.5) | 3 (10) | 7 (11.7) |
| Traumatic fracture | 1 (3.3) | 2 (6.7) | 3 (5) |
| Calcium and vitamin D supplement before treatment | 14 (46.7) | 8 (26.7) | 22 (36.7) |
| Calcium supplementation only after treatment | 29 (96.7) | 30 (100) | 59 (98.3) |
| Calcium and vitamin D supplementation after treatment | 22 (73.3) | 19 (63.3) | 41 (68.3) |
Abbreviation: SRNS, Steroid-Resistant Nephrotic Syndrome; SDNS/FRNS, Steroid-Dependent / Frequent-Relapsing Nerphrotic Syndrome.
aValues are expressed as No. (%).
Table 3 shows duration of therapy with steroid and other immunosuppressive drugs in both study groups.
| Variables | SDNS/FRNS, y | SRNS, y |
|---|---|---|
| Steroid | 4 ± 2.5 | 3.9 ± 2.7 |
| Azathioprine | 0.5 ± 1 | 0.34 ± 0.65 |
| Cyclophosphamide | 0.9 ± 0.16 | 0.2 ± 0.28 |
| Mycophenolate | 0.18 ± 0.57 | 0.32 ± 0.8 |
| Cyclosporine | 0.32 ± 1.2 | 1.5 ± 1.3 |
| Levamisol | 0.72 ± 1.1 | 0.3 ± 0.9 |
Abbreviations: SRNS, Steroid-Resistant Nephrotic Syndrome; SDNS/FRNS, Steroid-Dependent / Frequent-Relapsing Nerphrotic Syndrome.
Table 4 shows laboratory results in both study groups in comparison to control group. Serum OC levels are significantly higher in both SDNS/FRNS and SRNS than in control group, with P value 0.02 and 0.01, respectively.
| Variables | Study Group | Control Group | P Value |
|---|---|---|---|
| Calcium, mg/dL | 9.8 ± 0.7 | ||
| SDNS/FRNS | 8.9 ± 0.8 | 0.0001** | |
| SRNS | 8.9 ± 0.8 | 0.0001** | |
| Phosphorous, mg/dL | 4.2 ± 0.7 | ||
| SDNS/FRNS | 4.1 ± 0.8 | 0.6 | |
| SRNS | 4.3 ± 0.8 | 0.8 | |
| ALP, U/L | 217 ± 103 | ||
| SDNS/FRNS | 273 ± 120 | 0.63 | |
| SRNS | 293 ± 153 | 0.02* | |
| OC, µg/L | 17.7 ± 6.6 (19.57/ 12.62 - 24.04) | ||
| SDNS/FRNS (median/ IQR) | 26.2 ± 17.6 (11.30/ 6.85 - 27.24) | 0.02* | |
| SRNS (median/ IQR) | 26 ± 16.3 (11.56 / 4.49 - 28.29) | 0.01* | |
| BUN, mg/dL | 15.5 ± 8 | ||
| SDNS/FRNS | 13.2 ± 6.9 | 0.25 | |
| SRNS | 13.9 ± 9.6 | 0.5 | |
| Creatinine, mg/dL | 0.55 ± 0.2 | ||
| SDNS/FRNS | 0.53 ± 0.2 | 0.78 | |
| SRNS | 0.6 ± 0.2 | 0.5 |
Abbreviations: ALP, Alkaline Phosphatase; IQR, Interquartile Range; OC, Osteocalcin, BUN, Blood Urea Nitrogen; SRNS, Steroid-Resistant Nephrotic Syndrome; SDNS/FRNS, Steroid-Dependent / Frequent-Relapsing Nerphrotic Syndrome.
When we divided the 60 patients in our study according to the state of remission or relapse at the time of the study, we found that mean serum OC level in relapsed patients was 20.3 ± 16.6 ng/mL which was lower than the level in patients in remission (30.2 ± 16 ng/mL). This difference is statistically significant (P = 0.001).
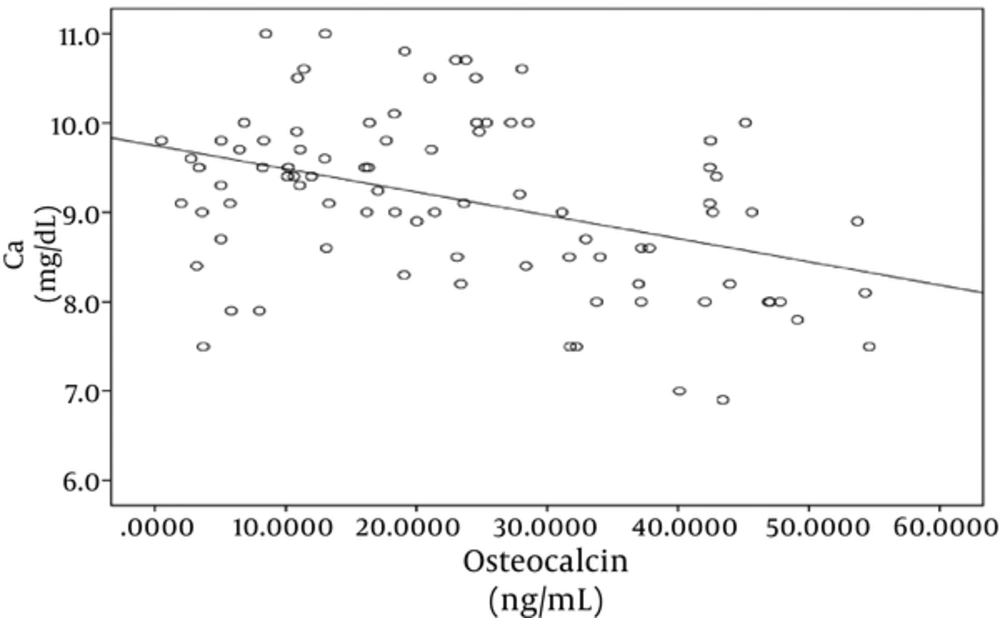
In both case and control groups, there was statistically significant negative correlation between OC and serum calcium (r = -0.4, P = 0.0001) as shown in Figure 1, while there was no significant correlation between OC and phosphorus and alkaline phosphatase.
There was a negative correlation between height for age percentile and number of relapses (r = -0.25, P = 0.05). There is also a negative correlation between height for age percentile and duration of steroid therapy (r = -0.27, P = 0.05). There were also negative correlations between height for age percentile and cyclophosphamide (r = -0.26, P = 0.044) and cyclosporine duration of treatment (r = -0.3, P = 0.02).
4. Discussion
Long-term use of corticosteroids in children with NS may lead to bone metabolism disorders, whose mechanisms are underlain by both steroid therapy and biochemical changes due to proteinuria that influences bone turnover and mineralization (10).
Raisz (2005) (11) stated that glucocorticoids (GC) modify the strictly regulated balance of bone metabolism. Secondary osteoporosis is caused by a relative increase of bone resorption over bone formation (12). This results in reduced osteoblastic differentiation and osteoblastic activity by for example suppressing the synthesis of type I collagen, a major component of bone matrix, as well as lowering osteoblastic activity by accelerating apoptosis. Depressed bone formation is considered to be the main skeletal target of GC action (13).
Other contributing factors are decrease of muscle mass, sex hormone levels and a suppression of the somatotrophic axis accelerate the onset of GC–induced osteoporosis (14).
During the current study, male patients represented percentage of 66.7% and 63.3% of patients with SDNS/FRNS and SRNS respectively. In agreement with our study, Hammad et al. (2013) (15) found that 65% of SRNS were males and Shah et al. (2015) (16) found that 66.7% of their SRNS patients were males.
There was statistically higher weight for age percentile in SDNS/FRNS patients compared to control group (P = 0.005); while, there was no statistical difference between SRNS patient and control group. In both SDNS/FRNS and SRNS, we also found that BMI was higher in both patients groups when compared to control group (P = 0.0001 and 0.0001, respectively).
Moon et al. (2014) (17) found that the children with NS were heavier than the controls (P = 0.022) and had greater body fat percentage SDS (P = 0.008).
We found that both of our patients groups (SDNS/FRNS and SRNS) showed lower height for age percentile compared to control group (P = 0.017 and 0.00 respectively). In addition, we found that 50% of our patients were below 5th percentile for height.
In our study, we found that height of all nephrotic patients was negatively correlated to duration of steroid, cyclophosphamide and cyclosporine therapy (P = 0.05, P = 0.044 and 0.02 respectively). The explanation of this may be due to the association between the several prolonged and repeated courses of steroids therapy in combination with cyclophosphamide and cyclosporine. In addition we found that there was statistically negative correlation between height for age percentile and number of relapses (P value = 0.05).
This matched with what Ribeiro et al. (2015) (18) noted. They found that growth and spine bone mineral density (BMD) were both negatively associated with the cumulative dose of steroids (P = 0.001 and P = 0.037, respectively).
In contrary, Leroy et al. (2009) (19) followed 64 children with SDNS receiving long-term cyclosporine and steroid therapy which were retrospectively analyzed. During the 10-year follow-up period, height standard deviation score (HSDS) remained in the normal range in 47 patients but was below -2 SD in 17 patients.
Only 3 (5%) patients of our study group had bone fractures (proved by plain X-ray) during the course of the disease and under treatment of steroids. By history, two of them were on irregular dose of calcium only without vitamin D and one did not receive any calcium or vitamin D supplement treatment. This was found when Phan et al. (2014) (20) studied 54 children followed to 12 months after steroid initiation. Three of 54 children with radiographs had incident vertebral fracture at 1 year.
There were significantly lower calcium levels in both SDNS/FRNS and SRNS patient groups compared to the control group (P = 0.0001, 0.0001, respectively). As regarding serum alkaline phosphatase (ALP) in our study, we found higher ALP levels in both SDNS/FRNS and SRNS patients compared to control group but with statistical significance for only SRNS patients (P = 0.02).
Osteocalcin is a non-collagenous protein of bone matrix, and it is a good marker of bone formation and a sensitive indicator of inhibitory effects of corticosteroids (21). It is believed that it acts on the bone matrix to regulate mineralization (22).
In our study, we found higher OC levels in both SDNS/FRNS and SRNS patient groups compared to the control group (P = 0.02 and 0.01 respectively). We also found that OC levels were negatively correlated to serum calcium levels of the whole patients (P value = 0.0001).
The studies in adults have shown that a single oral dose of 2.5 mg prednisone exerts an almost immediate effect on the serum OC level (23). Biyikli et al. (2004) (24) found a negative influence of long term corticosteroid therapy on the OC level in children with nephrotic syndrome.
When we divided the 60 patients in our study according to the state during the study period if they were in remission or relapse, we found that mean serum OC level in relapsed patients was 20.3 ± 16.6 ng/mL which is lower than its mean level in patients in remission (30.2 ± 16 ng/mL). This difference is statistically significant (P = 0.001).
In agreement with our study, Mohamed and Abdel-Latif (2011) (2), studied 30 NS patients in a case-control study and they found that children with NS, whether in one group or classified into frequent relapse and infrequent relapse, had significantly higher serum OC than controls (P value = 0.0001).
In another study done by Panczyk-Tomaszewska et al. (25), 25 children with idiopathic NS were observed over 1-year, they were treated with prednisone (a mean starting dose of 0.7 ± 0.5 mg/kg/48 h) and vitamin D (800 IU/24 h) and all tests weres done during remission. It was found that serum calcium, phosphorus, alkaline phosphatase and OC were inappreciably different at the beginning and end of the therapy time.
A major limitation of this study was the small sample size and lack of follow up. Also, in our study we did not do dual-energy X-ray absorptiometry (DEXA) scan or bone ALP which are not available in our hospital, plus these are too expensive.
4.1. Conclusion
Patients having nephrotic syndrome on long-term steroid use (SDNS/FRNS and SRNS) are at higher risk of bone metabolism disorders. There is a higher bone turnover in patients treated with high doses of steroids which may affect patient’s growth and height, so it is important to follow up the growth parameters including height, weight and BMI in all patients with nephrotic syndrome. Height as a growth parameter is more affected when recurrent relapses occur with multiple courses of steroid therapy. Careful evaluation of BMD and early prophylactic supplementation with calcium and vitamin D and regular follow up of its level with increasing the dose if there is any abnormality are necessary. Use of OC as screening tool is recommended for bone turnover while patients on steroids.