1. Background
Approximately 60% of term and 80% of premature infants are hospitalized for hyperbilirubinemia in the first week of life. Hyperbilirubinemia is the most common cause of hospitalization in infancy. Physiological icterus is a common cause of infantile hyperbilirubinemia which is diagnosed by ruling out other important etiologies such as hemolysis, infections, and metabolic disorders (1-3). Hyperbilirubinemia is the most common cause of readmission in early infancy (4, 5). In 2% of mature infants, bilirubin levels can reach more than 20 mg/dL which requires therapeutic action and may result in complications such as kernicterus and neurological damage (6).
The aim of managing hyperbilirubinemia is to prevent neurological damage. The most prevalent therapeutic method for managing infantile icterus and preventing its complications is phototherapy, which has been applied for decades as a safe method (7). However, higher levels of bilirubin may require a blood transfusion.
Previous studies have suggested medications such as metalloporphyrins, phenobarbital, clofibrate, and IVIG for management of hyperbilirubinemia (8-12). Active charcoal, agar, and cholestyramine are also used as adjuvants in decreasing bilirubin levels (13). Probiotics are microorganisms which can decrease the transit time of materials in the intestines (14). A few studies assessing the effect of probiotics on decreasing bilirubin levels have reported decreases in the required length of phototherapy (15-17).
2. Objectives
This study aims to assess the adjunctive effect of probiotics on decreasing hospitalization time in order to address the related issues of the high frequency of infants hospitalized with hyperbilirubinemia and the high costs of treatment.
3. Methods
This randomized, controlled clinical trial was approved by the ethics committee of Baqiyatallah University of Medical Sciences and registered with the Iranian registry of clinical trials) Reference Number: IRCT2015021817413N11). Figure 1 shows a flowchart of the trial. Patients with hyperbilirubinemia who were admitted to the Neonatal Intensive Care Unit of Baqiyatallah Hospital from March to June 2014 were enrolled in the study. A written informed consent form was obtained from all of the guardians.
Infants with a gestational age of more than 35 weeks, birth weight of more than 2500 grams, bilirubin level of more than 15 mg/dL, and direct bilirubin level of less than 1.5 mg/dL were included in the present study. Infants who were less than two days of age, were ill or septic, had a positive Coombs test, were undergoing blood transfusion or IVIG, were receiving phenobarbital therapy, required serum therapy, or whose parents were not willing for their children to participate in the experiment were excluded from the study. The patients who met the inclusion criteria were randomly assigned to two groups (control and probiotic) using a random-number tabulation. Serum therapy and taking phenobarbital, bilinaster, or manna was prohibited during the study.
The probiotic group was treated with half of a capsule of Prokid probiotic (Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium bifidum, and Lactobacillus rhamnosus). The control group used a placebo which was half of a capsule filled with a probiotic-free formula the same color as the Prokid probiotic. The probiotic and placebo capsules were prescribed once a day before breastfeeding. Neither the parents nor the nurse were informed whether the administered drug was a placebo or not.
The patients’ bilirubin levels were measured using serum samples prior to admission and after treatment. All of the patients with bilirubin levels higher than 18 mg/dL underwent intensive phototherapy. Those with bilirubin levels between 14 mg/dL and 18 mg/dL underwent 8-lamp phototherapy. Those with bilirubin levels less than 14 mg/dL were treated with 4-lamp phototherapy. Infants with bilirubin levels less than 10 mg/dl during the first week and less than 11 mg/dl after the second week were discharged. The duration of the phototherapy, the blood groups of mothers and infants, and the patients’ bilirubin levels before and after phototherapy, direct Coombs test results, and levels of hemoglobin, G6PD, and reticulocytes were recorded.
Data were analyzed using SPSS software version 20 (SPSS Inc., Chicago, IL) for Microsoft Windows. The normal distributed variables (approved by a 1-sample Kolmogorov-Smirnov test) were compared using an independent sample t test between the groups. A Mann-Whitney U test was used to compare between the groups for variables that were not of a normal distribution. The chi-square test was used to compare categorical variables in the two groups and the Fisher test was used for all other conditions. A P-value of less than 0.05 was considered to be statistically significant.
4. Results
Data from 92 patients with a mean age of 5.25 ± 2.35 days underwent analysis. Table 1 summarizes the demographic and laboratory characteristics of the patients.
| Variable | Probiotic (n = 45) | Control (n = 47) | P Value |
|---|---|---|---|
| Mean age, d | 5.31 ± 2.19 | 5.19 ± 2.51 | 0.81 |
| Male, No. (%) | 17 (37.7) | 23 (48.9) | 0.2 |
| Severe G6PD deficiency, No. (%) | 2 (4.4) | 1 | 0.61 |
| Mean reticulocyte count | 1.77 ± 1.96 | 1.42 ± 1.56 | 0.34 |
| Mean hemoglobin level, mg/dL | 16.29 ± 1.31 | 16.98 ± 1.70 | 0.03 |
The distribution of birth weight between the two groups is summarized in Table 2. Most of the infants (52.2%) weighed between 3000 and 3500 grams. There was no significant difference between the two groups for distribution of birth weight (P = 0.18).
| Groups | Birth Weight (Grams) | Total | P Value | ||
|---|---|---|---|---|---|
| 2500 - 3000 | 3000 - 3500 | > 3500 | |||
| Control | 15 (16.3) | 29 (31.5) | 3 (3.3) | 47 (51.1) | 0.18 |
| Probiotic | 14 (15.2) | 19 (20.7) | 12 (13) | 45 (48.9) | |
| Total | 29 (31.5) | 48 (52.2) | 15 (16.3) | 92 (100) | |
aValues are expressed as No. (%).
Most of the infants in both the probiotic (74%) and control (79%) groups were born by normal vaginal delivery. Eighty-five of the infants (92.4%) were being breastfed and 7 of the infants (7.6%; 2 in the probiotic group and 5 in the control group) received both breast milk and formula. There was no significant difference between the two groups with respect to the type of nutrition (P = 0.43).
On the first day of the study (before intervention), the 92 infants had a mean bilirubin level of 16.70 ± 3.07 mg/dL, with a mean of 16.46 ± 2.33 mg/dL in the control group and 16.95 ± 2.67 mg/dl in the probiotic group (P = 0.35). Table 3 shows the mean bilirubin level trend in the patients before and after intervention. The day after intervention, the probiotic group had a significantly lower bilirubin level (P = 0.001). The mean bilirubin level decrease was 4.80 ± 1.76 in the probiotic group and 2.76 ± 1.29 in the control group the day after intervention (P < 0.001). Most of the infants (84.7%) in the probiotic group were discharged two days after intervention, but 32 of the infants (69.5%) in the control group were still under treatment on the third day. At the time of discharge, infants had a mean bilirubin level of 9.72 ± 0.96 mg/dL in the probiotic group and 9.38 ± 0.71 mg/dL in the control group (P = 0.053).
| Day | Probiotic | Control | P Value | ||
|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | ||
| 1 | 16.95 ± 2.67 | 45 | 16.46 ± 2.33 | 47 | 0.35 |
| 2 | 12.12 ± 2.16 | 45 | 13.71 ± 2.13 | 47 | 0.001 |
| 3 | 10.14 ± 1.17 | 38 | 10.91 ± 1.88 | 47 | 0.026 |
| 4 | 10.10 ± 1.11 | 7 | 10.15 ± 1.49 | 32 | 0.930 |
| 5 | 9.8 | 1 | 10.29 ± 1.41 | 10 | 0.749 |
| 6 | - | 0 | 10.76 ± 1.26 | 3 | - |
| 7 | - | 0 | 10.6 | 1 | - |
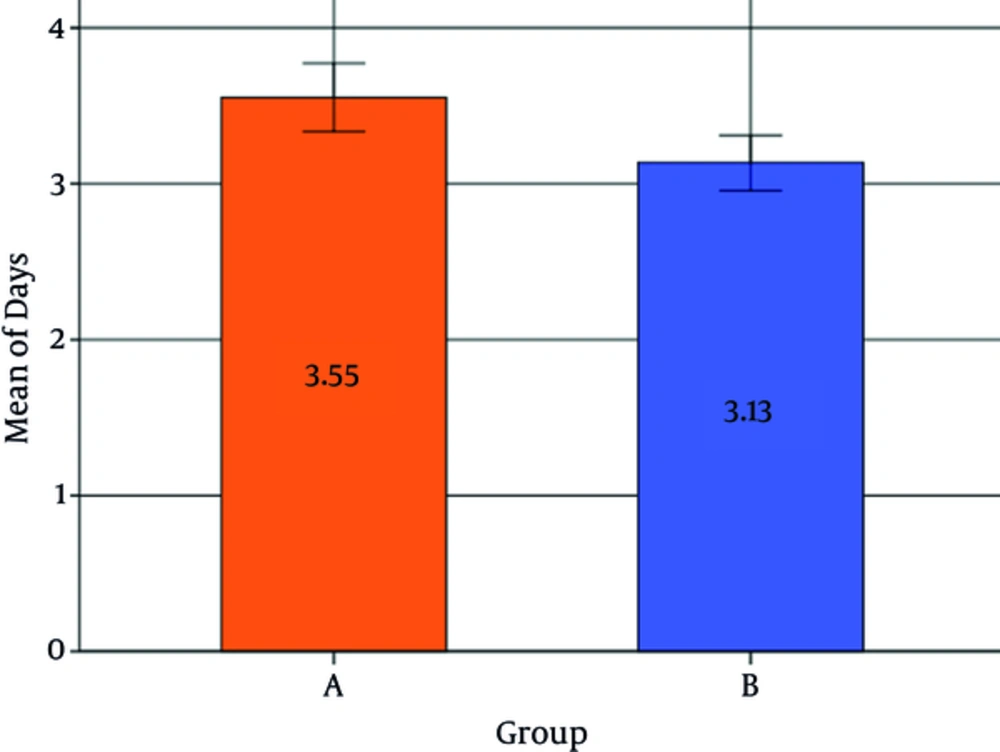
The average duration of hospitalization was 3.34 ± 0.70 days overall, with an average of 3.55 ± 0.74 days for the control group and 3.13 ± 0.70 days for the probiotic group (Figure 2). The probiotic group had a significantly lower hospitalization stay in comparison to the control group (P < 0.001, [CI = 95%, -1.31 to - 0.64]). Female patients had on average a significantly lower hospitalization duration in comparison to male patients (P = 0.005). No significant association was found between birth weight or age and the length of hospitalization duration (P > 0.05) (Table 4).
| Age | Birth Weight | ||
|---|---|---|---|
| Days | Spearman’s rank correlation | 0.070 | -0.059 |
| P Value | 0.507 | 0.577 | |
| Frequency | 92 | 92 |
5. Discussion
In the present study we found that the adjuvant prescription of probiotics with phototherapy reduces the hospitalization duration in infants with hyperbilirubinemia. Only one similar study has been conducted with very low birth weight infants and no studies have yet been conducted with term infants (15).
The aim of hyperbilirubinemia management is to prevent the indirect bilirubin levels from reaching the point at which neurotoxicity may occur. Possible complications of hyperbilirubinemia include deafness and kernicterus. Phototherapy is the treatment of choice for infant hyperbilirubinemia, and was administered equally for the infants in both groups in the present study (18). However, long-term phototherapy is associated with complications such as erythematous rashes, diarrhea, and hyperthermia.
The social and economic burdens of hospitalization have led to the application of nursery services at home such as at-home phototherapy. Hospitalization in a government hospital for a mature, healthy infant costs three times more than at-home phototherapy, and the same treatment is six times more expensive in non-government hospitals. Finding a way to decrease the hospitalization duration has several benefits including lower expenses and more emotional contact between mother and child (19-21). Thus, finding a way to reduce the phototherapy period is necessary in both the economic and medical aspects.
The prescription of a variety of drugs such as clofibrate and phenobarbital has been proposed for reducing hospitalization duration (18). However, phenobarbital has been shown to have no significant clinical effect on treating hyperbilirubinemia except the dominant type of Crigler-Najjar syndrome (10). Active charcoal, agar, and cholestyramine are non-absorbable oral agents which decrease enteric absorption of bilirubin and its serum level by reducing the enterohepatic cycle (13). Caglayan et al. demonstrated that a combination of agar and phototherapy was more effective than phototherapy alone (22).
An increase in bowel transit, which was induced by probiotics in our study, is one of the potential ways of decreasing treatment duration of physiological hyperbilirubinemia, although laxatives were shown to be not effective in managing infant hyperbilirubinemia in another study (10).
The purpose of probiotics is to increase the number of beneficial bacteria in the bowels. No adverse effects from the administered probiotics were found in the present study. Recently, probiotics have been shown to be useful for treating gastroenteritis by slowing bacterial growth (23). They can decrease the passing time of materials in the bowels, regulate smooth muscle contraction, and increase the reproduction of enterocytes (14).
Demirel et al. evaluated the effect of a Saccharomyces boulardii probiotic supplement on the course of hyperbilirubinemia and the duration of treatment in 179 very low birth weight infants in a clinical trial. They reported that the duration of phototherapy was significantly lower in the probiotic group in comparison to the control group which is in accordance with the present study. The incidence of feeding intolerance was also lower in their study group (15).
The present study has some limitations. No long-term follow-up regarding the possible side effects of the probiotics was performed for the patients. In addition, the frequency of defecation in both groups is another factor that should be considered in future studies.
In conclusion, our findings suggest that probiotics may be beneficial as an adjunct treatment for infants with hyperbilirubinemia by reducing the duration of hospitalization. Further in-depth studies are necessary to establish the effect of probiotics on the course of hyperbilirubinemia.