1. Background
Obesity is prevalent in the world and is a main common health problem especially in childhood (1, 2). In Europe, similar to other developed countries, the prevalence of childhood overweight and obesity is between 20% to 40% (3). There has also been a remarkable increase of health complications related to excessive adiposity, including dyslipidemia, hypertension, abnormal blood glucose, psychological depression, and decreased quality of life in childhood (2, 4, 5). Illnesses being dangerous for human health, have always engaged the minds of researchers. According to previous studies, cardiovascular diseases (CVDs) kill 12 million people yearly (6). Researches show that obesity is a risk factor for CVDs (7).
Lipid profile presented as an indicator of CVDs, increased LDL-C and decreased HDL-C are the risk factors for CVDs. One of the most important strategies to ameliorate human health is controlling risk factors lipid profile and childhood obesity. Lipid profile disorders can lead to development of CVDs (4). Several researches have shown that regular long term exercises with low to middle intensity can improve lipid profile risk factors (8, 9). By contrast, some other researchers found no improvement in lipid profile after the endurance exercise training in childhood (10-12). Researchers suggested that endurance exercise training compared with resistance exercise training can increase fitness to perform daily activity and has more useful effects for individuals susceptible to cardiovascular diseases. They reported that if intensity and duration of endurance exercise training are suitable, it can be more effective in the enhancement of HDL-C levels than resistance exercise training (5).
Several studies have concerned the impact of regular physical activity on the improvement of diabetes and hypertension in childhood (7, 10). One of the important parts in this field is the impact of exercise on blood glucose control and blood pressure in diabetic patients and obese children. It has been suggested that exercise decreases blood pressure and serum glucose. Performed ordinarily, exercise training can decrease blood pressure by an average of 8 - 10 mmHg (7, 10).
Physical inactivity leads to metabolic and structural alterations among children and healthy individuals, such as increased insulin resistance, hypertension, and changes in plasma lipid profile (13). High blood pressure and damaged lipid profile are among the risk factors of cardiovascular illnesses. These are also considered as elements of metabolic syndrome (7, 14).
2. Objectives
Previous researches evaluated the effect of endurance exercise training with contradictory findings. And few studies have been done on the effect of endurance exercise training on blood glucose, blood pressure, ankle-brachial index, and lipid profile in male obese children. This study aimed to identify which of the methods, endurance, resistance, or combined training affects cardiovascular risk factors in male obese children.
3. Methods
3.1. Participants
Elementary schools of Yasuj city, Western Iran, comprise totally 8987 male students aged 8 - 12 years, 105 of whom had a BMI between 30 and 35 kg/m2 (Z-score above +2). Of these, 60 children volunteered to participate in the study. They were randomly divided into four groups (each group consisting of 15 participants) for resistance exercise training (RET), endurance exercise training (EET), or combined exercise training that was a combination of endurance plus resistance exercise training (CET), and controls (C).
Parents of the children were in advance informed about the aim and design of the research, and those who accepted to take part signed the consent form. Subjects were given medical examination before beginning the study. During the study weight and height of participants were regularly assessed and a physician used to examine their physical condition. Characteristics of the participants are given in Table 1.
| Characteristics | Mean ± SD |
|---|---|
| Age (yr) | 10.05 ± 1.41 |
| Weight (kg) | 71.21 ± 11.02 |
| Height (m) | 1.47 ± 0.09 |
3.2. Antropometric Measures
Standing height was measured using a stadiometer (DETECTO, Model 3PHTROD-WM, USA) with a fixed vertical backboard and an adjustable head piece. Weight was measured by Omron scale (Model HBF 516, China) without shoes and excess clothing.
3.3. Exercise Training Protocol
EET included 15 minutes warm-up with stretching movements and jogging and then 4 to 7 sets of continuous running. Each set lasted 5 minutes of continuous running with 1 minute rest between sets. Running intensity was between 50% to 75% target heart rate calculated with the Karvonen formula. In order to observe the overload principle, the researchers increased the number of running sets and intensity rate every two weeks. At the end of each training session, cool down for 10 minutes was performed by slow running and stretching motions. Exercise training intensity was achieved by following Karvonen formula:

HRmax was calculated as [208 - 0.7 (age)] (18) and HRr was achieved as the mean of 3 evaluations in the morning.
RET schedule contained circuit exercise training (exercise training with weights in 5 stations) with 1 minute rest between sets performed in a station exercise in 3 sets with 8 repetitions and 1 minute rest between sets. Also, resistance training intensity was 50% 1RM (1 repetition maximum) at the start of training period and 75% 1RM at the end of training period. Aim of RET was the use of various muscles at diverse stations. Therefore, RET program was aimed to improve cardiovascular endurance and to strengthen the muscles (3). RET schedule was done for 8 weeks and 4 times per week. Warm-up was performed for 15 minutes per session that included stretching movements in the body’s major muscles and jogging, then RET program continued for 25 - 40 minutes, followed by cool-down with jogging and stretching movements for about 10 minutes. RET program included quadriceps muscles contraction (knee extension) with machine, hamstring muscles contraction (knee curl) with machine, biceps curl with dumbbell, triceps extension with dumbbell, and butterfly exercise with machine.
Exercise training intensity used in RET was 50% - 75% of 1RM (one maximum repetition). 1RM is the maximum weight that a person would lift and was calculated by the Brzycki’s equation (15):

The exercise protocols were designed based on the principle of overload. Therefore, exercise training intensity or repetitions of movements were added every two weeks (10).
The CET program included 2 sessions of RET and 2 sessions of EET. Group C had no exercise training during this study. All exercise training protocols were supervised by sport physiologists specialists. Exercise training location was the sport champions base of Yasuj.
3.4. Blood Sampling and Assessment
Blood sampling was performed 24 hours before the exercise training period and 48 hours after the last session in four groups at the laboratory of Shahid Beheshti hospital, Yasuj. blood sampling, the participants no intensive activity. Blood was taken from the right brachial artery up to 10 mL after 14 hours of night fasting. The plasma samples were gained after centrifuging at 4°C with 3000 rpm for 15 minutes. TC, HDL-C, and TG concentrations were calculated with enzymatic ways using an automated clinical chemistry analyzer (Dimension RxL Max, Siemens Healthcare Diagnostics, Germany). LDL-C was calculated by the Friedewald, Levy, and Fredrickson equation (LDL = TC - HDL - TG/0.5) (14). VLDL levels were measured based on the relationship VLDL = TG/5.
Fasting blood glucose (FBG) concentration was measured with a modified hexokinase enzymatic method (7020 clinical analyzer, Hitachi, Tokyo, Japan).
3.5. Resting Heart Rate
Resting heart rate was examined by a stethoscope (Hi-tec-Canada) at rest as the mean value of three measurements in the morning for each participant.
3.6. Ankle-Brachial Index (ABI) Measurement
ABI is applied to determine the peripheral arterial illness (PAD). PAD is the narrowing of the peripheral arteries in legs, stomach, arms, and most commonly in the arteries of legs. After the patients had rested in the supine position for 10 minutes, the ABI was calculated by measuring the systolic blood pressure from both brachial, dorsalis pedis and posterior tibial arteries. The systolic pressure was measured with a handheld 5- or 10-mHz Doppler instrument (Toshiba SSA-380A, PowerVision, Tokyo, Japan). The ABI rate was gained dividing ankle arterial systolic pressure by the brachial arterial systolic pressure (6).
3.7. Cardiovascular Fitness (VO2max) Measurement
VO2max (maximal oxygen consumption) is the maximum rate of oxygen consumption in 1 kilogram of muscle tissue in one minute. VO2max was calculated with PACER fitness test as follows: mark off a 20-meter course that allows enough space for each subject to run freely. Each subject should then run the full 20 meters after beep sound was played, touch the line at the end of the 20 meters with their foot and wait for the CD to beep. They follow doing this until subjects cannot get to the next line before the CD beeps. Once subject fails to reach the other end before it beeps twice, test is finished. Each 20-meter run is calculated as one lap. Their total laps are their total score. The test scores are entered into the Fitness Gram software to estimate the VO2max. Reliability of the PACER has been illustrated in children aged 6 to 16 years (10), whilst validity is shown in children aged 8 to 19 years (10, 16).
3.8. Statistical Analysis
The effects of the training programs on measured parameters were evaluated by ANOVA in repeated measures with 2 × 4 (2 times of parameters measurement multiplied by 4 groups) design. The normality of data and equality of variances were respectively calculated by the Kolmogrov-Smirnov test and Levene’s test. Data were presented as mean ± SD. Data analyses were conducted with SPSS software (version 21). The values were considered significant when P < 0.05.
4. Results
Eight weeks of exercise training in EET, RET and CET groups compared to group C, caused a significant increase in HDL and a significant reduction in TC, TG, LDL, VLDL, TC/HDL and LDL/HDL ratio (P < 0.001) (Table 2). Moreover, results of one way ANOVA showed that EET was more effective in improving these parameters in comparison to RET and CET. However, none of EET, RET and CET compared to group C had a significant effect on the rate of left and right ankle-brachial index (ABI) (P > 0.05). The one way ANOVA showed also that EET was more effective in improving these parameters in comparison with RET and CET.
| Variables | Group | Pre-Training | Post-Training | T | T × G | G |
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | EET | 106.20 ± 19.08 | 88.13 ± 11.06 | 81.70b | 11.09b | 1.94c |
| RET | 106.26 ± 19.31 | 94.86 ± 13.86 | ||||
| CET | 107.06 ± 20.70 | 91.13 ± 11.31 | ||||
| C | 105.06 ± 16.85 | 105.53 ± 16.56 | ||||
| TC (mg/dL) | EET | 243.46 ± 65.71 | 155.33 ± 50.60 | 323.26b | 40.27b | 3.28c |
| RET | 242.33 ± 52.99 | 173.73 ± 42.63 | ||||
| CET | 242.86 ± 57.09 | 163.86 ± 48.87 | ||||
| C | 241.06 ± 46.05 | 243.33 ± 46.81 | ||||
| TG (mg/dL) | EET | 263.86 ± 56.32 | 168.26 ± 50.33 | 139.84b | 19.27b | 4.57c |
| RET | 265.33 ± 46.39 | 193.33 ± 41.52 | ||||
| CET | 259.40 ± 55.65 | 177.13 ± 49.35 | ||||
| C | 269.33 ± 56.65 | 274.73 ± 51.93 | ||||
| LDL (mg/dL) | EET | 151.96 ± 56.67 | 73.08 ± 39.25 | 264.99b | 31.78b | 3.79c |
| RET | 148.33 ± 48.23 | 88.26 ± 35.86 | ||||
| CET | 152.38 ± 50.12 | 82.37 ± 41.97 | ||||
| C | 146.06 ± 49.27 | 146.85 ± 45.19 | ||||
| VLDL (mg/dL) | EET | 52.77 ± 11.26 | 33.65 ± 10.06 | 139.84b | 19.27b | 4.54c |
| RET | 53.06 ± 9.27 | 38.66 ± 8.30 | ||||
| CET | 51.88 ± 11.13 | 35.42 ± 9.87 | ||||
| C | 53.86 ± 11.33 | 54.94 ± 10.38 | ||||
| HDL (mg/dL) | EET | 38.73 ± 3.67 | 48.60 ± 4.20 | 203.48b | 23.59b | 2.03c |
| RET | 40.93 ± 5.21 | 46.80 ± 5.46 | ||||
| CET | 38.60 ± 4.80 | 46.06 ± 5.27 | ||||
| C | 41.13 ± 4.34 | 41.53 ± 5.26 | ||||
| LDL/HDL (mg/dL) | EET | 3.95 ± 1.49 | 1.49 ± 0.78 | 291.33b | 34.70b | 2.90c |
| RET | 3.65 ± 1.18 | 1.91 ± 0.81 | ||||
| CET | 4.05 ±1.46 | 1.86 ± 1.00 | ||||
| C | 3.61 ±1.37 | 3.61 ± 1.26 | ||||
| TC/HDL (mg/dL) | EET | 6.32 ±1.72 | 3.18 ± 0.97 | 391.53b | 47.6b | 2.58c |
| RET | 5.98 ±1.36 | 3.74 ± 0.97 | ||||
| CET | 6.43 ±1.79 | 3.65 ± 1.26 | ||||
| C | 5.93 ±1.41 | 5.95 ± 1.40 | ||||
| ABIleft (mmhg) | EET | 1.08 ±0.05 | 1.04 ± 0.12 | 1.65 | 0.81 | 2.60 |
| RET | 1.00 ±0.12 | 0.96 ± 0.05 | ||||
| CET | 1.00 ±0.14 | 0.94 ± 0.09 | ||||
| C | 0.98 ±0.13 | 1.01 ± 0.13 | ||||
| ABIright (mmhg) | EET | 1.07 ±0.10 | 1.02 ± 0.14 | 2.62 | 0.77 | 0.32 |
| RET | 1.06 ±0.10 | 1.02 ± 0.11 | ||||
| CET | 1.03 ±0.10 | 0.99 ± 0.13 | ||||
| C | 1.01 ±0.14 | 1.03 ± 0.15 |
Abbreviations: ABIleft, left ankle-brachial index or left ankle blood pressure/left brachial blood pressure ratio; ABIright, right ankle-brachial index; CET, combined exercise training; C, control; G, groups; T × G, times × groups endurance exercise training; HDL, high density lipoprotein; LDL, low density lipoprotein; LDL/HDL, LDL/HDL ratio as a coronary risk factor; VLDL, very low density lipoprotein; RET, resistance exercise training; TC, total cholesterol; TG, triglyceride; TC/HDL, TC/HDL ratio as a coronary risk factor; T, times.
aValues are shown as mean ± standard deviation.
bP < 0.001.
cP < 0.05.
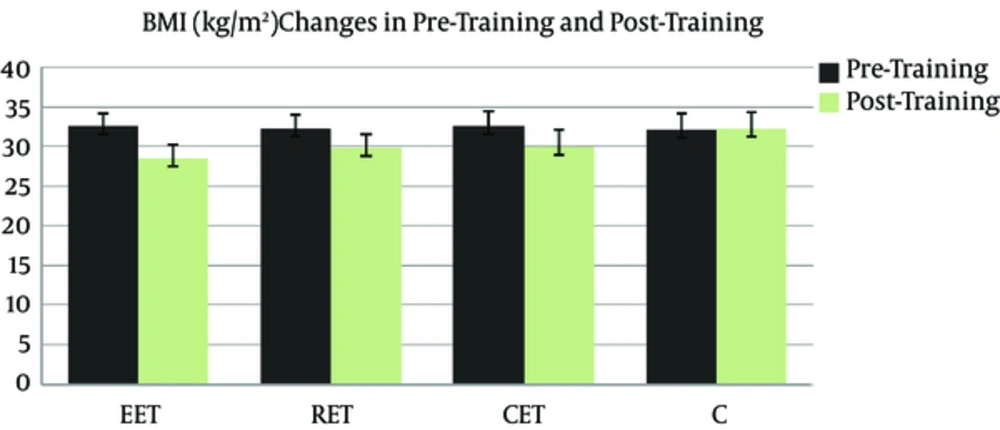
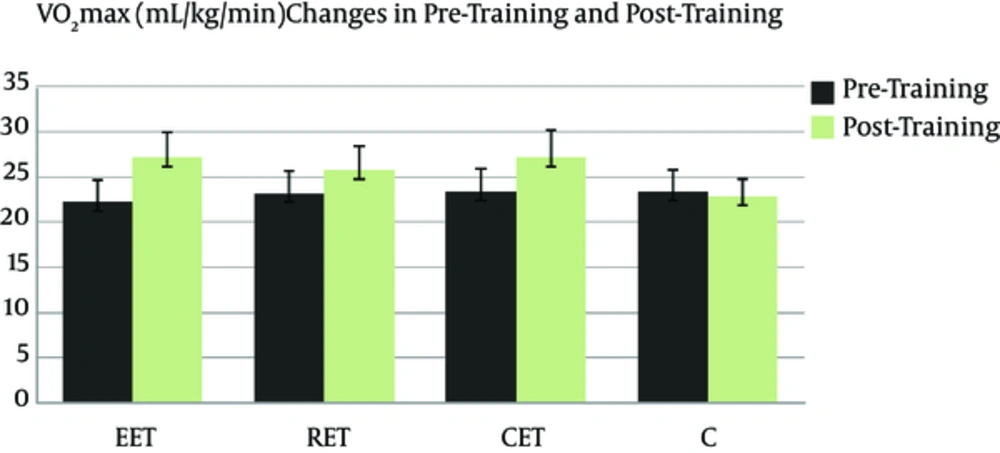
Figures 1 and 2 show BMI (kg/m2) and VO2max (ml/kg/min) changes in pre-training and post-training in all groups. It can be seen that 8 weeks of exercise training in EET, RET and CET groups compared to group C caused a significant increase of VO2max and a significant decrease in BMI (P < 0.001).
5. Discussion
The present study aimed to evaluate the effects of three methods of exercise training on cardiovascular risk factors in male obese children aged 8 - 12 years. Results showed that three types of exercise training significantly increased HDL-C, significantly decreased fasting glucose, BMI, TC, TG, LDL, VLDL, HDL, TC/HDL and LDL/HDL (P < 0.001). In Addition, the EET method compared to the RET and CET training methods, had a more significant effect on improving these parameters and did not have significant effect on the ankle-brachial index (ABI) (P < 0.05).
The results are consistent with the findings of previous studies indicating that orderly exercise is followed by decreased lipid profile (2, 17-21). By contrast, the results are inconsistent with the findings of some other studies (22). E Crimi et al reported the effect of two months of walking exercise on lipid profile and BMI in obese female children. Their findings showed that the BMI and risk factors of lipid profile reduced significantly, but HDL-C significantly increased (18). They suggested that an orderly exercise like walking may change the metabolism of lipoproteins and decrease the risk factors of CVDs (18). M Balas-Nakash et al reported that longer endurance exercises cause improvement of health status and decrease risk factors of CVDs. It is probably due to the use of fatty acids as fuel during exercise to produce muscle energy through increasing the level of catecholamines, cortisol, and growth hormones (19).
The present research indicates that endurance, resistance, and combined exercise trainings may significantly decrease LDL-C. At least two key enzymes exist in the metabolism of lipoproteins in relationship with exercise training (18). Lipoprotein lipase may have an important role in the rate of LDL-C to HDL-C alteration and its rate enhances endurance exercise training. Moreover, it seems that the enhanced level of HDL-C as a result of endurance exercise training, is because of the reduction of hepatic lipase activity, changes in different enzymes such as raise in lipoprotein lipase and lecithin cholesterol acyl transferase (LCAT) (23). Conversion of LDL-C to HDL-C is an important role of the hepatic lipase enzyme. The level of this enzyme may decline in persons who do exercise training, and being physically active may increase HDL-C concentration. These enzymatic alterations, which are caused by exercise training, ameliorate the serum lipoproteins (10, 23). Some studies suggest that orderly exercise usually ameliorates the serum lipoproteins in people with various ages and fitness levels (10, 24). However, there are various findings about what kind of lipid parameters are affected by intensity, type, and duration of exercise, dietary habits, baseline blood lipid profiles, physical specifications and cardiovascular fitness level of subjects.
VO2max levels of training groups significantly enhanced whereas no significant change was seen in the control group. It was shown that exercise training should be under anaerobic threshold in order to ameliorate lipid profiles (24). Several researchers have also stated that duration and intensity of exercise training, participants’ diet, lipid profile and weight condition of the participants lead to differences in lipid levels (25-28). HDL-C and coronary heart disease (CHD) have a powerful but negative correlation. Enhancing HDL-C concentrations after aerobic exercise training can partly illustrate protection from coronary heart disease (CHD) (28). In the current study, the HDL-C concentration compared to baseline significantly increased in children who did endurance exercise training.
Some studies illustrated that physical activity and exercise training may reduce BMI in children of 6 - 12 years of age (29). Past researches in overweight children after regular exercise training have presented improvements in serum insulin, while diet accompanied by aerobic exercise improved insulin resistance in overweight children. Exercise training is known to enhance insulin-receptor autophosphorylation, GLUT4 expression, and glucose transport. Therefore, the third main reason of insulin resistance is a sedentary lifestyle. A prompt effect of exercise training is an enhance in glucose transporters in muscle cell membranes, that secondarily improves insulin-mediated glucose disposal (30). It is expected that exercise training and desirable diets applied together would result in a more effective metabolism effect, as previously shown for glucose control improvement in diabetic patients (31).
Exercise training and physical activity decreased blood pressure levels in children, consistent with observational researches indicating that blood pressure levels were reversely correlated with the rate of physical activity in childhood (32-36). As CC Cesa et al have indicated, there are observational documents related to the existence of cardiovascular risk factors in childhood with a raised risk of atherosclerosis in adult life (37). Orderly exercise is related to a reduced CVDs risk in universes with and without type 2 diabetic disease (9, 12). This risk decrease likely happens through various functions in the body including improved lipid profile, glycemic control, and vascular function. Yet, beneficial effects of exercise training are not clear on ABI in PAD patients completely (10). Conversely, experimental researches have in general suggested that enhanced physical activity is related to higher rates for ABI in healthy persons (38, 39). The identification of a dose-response correlation between exercise training and the ABI in childhood remains difficult to achieve.
5.1. Conclusion
The findings of the present study show that three ways of regular exercise training performed in 8 weeks includeding resistance training (50% - 75% of 1RM) and endurance training (50% - 75% of target heart rate) had desirable effects on risk factors of BMI, serum glucose, and lipid profile. Therefore, three types of exercise training used in the present research, especially endurance training method could be considered as a suitable and non-medicational method to prevent and decrease the morbidity of CVDs and obesity-related disturbances in obese children. Future studies should focus on the long-term benefits of risk factors for metabolic syndrome after 8-weeks with diet and exercise training interventions in children.