1. Introduction
Obesity-related insulin resistance (IR) has become a major childhood health problem. In previous studies, the numbers of activated monocytes and macrophages have been reported to be elevated in obese patients. The correlation of body mass index (BMI) and the number of macrophages suggests that the fat tissue may be one of the main sources of blood monocytes (1). Activated monocytes invade the fat tissue and promote chronic inflammation by releasing cytokines, which may be linked to the mechanisms of obesity-induced hormone resistance (2, 3). Osteopontin (OPN) is a multifunctional pro-inflammatory cytokine involved in tissue transforming and also plays a role in the pathogenesis of atherosclerosis and diabetes. Many studies have described OPN as an essential regulator of fat inflammation and IR. OPN expression has been found to be strongly upregulated by 40 and 80 folds in fat tissue from diet-induced and genetically obese mice, respectively, and the analysis of fat cellular fractions has shown that most of the OPN supply in both humans and murinae with genetic and diet-induced obesity are adipose tissue macrophages (ATM) (4-6). OPN macromolecule expression may be iatrogenic from the spread of growth factors and cytokines, although the mechanisms of the upregulation remain not fully understood (7). In addition, OPN deficiency has improved glucose tolerance and reduced IR in mice, independent of body composition or energy expenditure (5). Insulin hormone resistance also plays a serious role in obesity. As the population gets heavier at younger ages, the age of onset of type 2 diabetes mellitus also decreases (8).
2. Objectives
In this study, we compared IR and serum OPN levels of obese dyslipidemic children to obese non-dyslipidemic children and expected higher levels of correlation of OPN and IR in the dyslipidemic group.
3. Methods
3.1. Study Design
A cross-sectional clinical trial was conducted in the outpatient clinic. Data related to age, anthropometric measurements, BMI, lipid profile, IR, liver function tests, and correlations between these parameters were assessed. Detailed physical examination and specified laboratory evaluations were performed for each patient. Standing height was measured to the nearest 0.1 cm with a Harpenden fixed stadiometer. Body weight (kg) was measured with a SECA balance scale to the nearest 0.1 kg, with each subject dressed similarly in lightweight clothing. The BMI was calculated by dividing the body weight (in kilograms) by the height (in meters) squared (kg/m2). A WHO chart was used for the BMI classification. Obesity was defined as BMI > the 97th percentile, which is the definition of the international obesity task force (9, 10). Children diagnosed with specific obesity-associated syndromes and with endocrine disorders were excluded from the study. No history or evidence of current systemic disease was present. None of the participants used any medication. Blood analyses were performed after fasting. The study was affirmed by the local ethical committee and written consent was obtained from the parents of the participants of both groups.
3.2. Sample Size Calculation
Previous studies have reported high serum OPN concentrations in diet-induced and genetically obese children (11). Based on previous findings, we assumed that the sample size of this study would allow us to detect differences in OPN levels between the two groups (α = 0.05, power = 80%). The α level was set at 0.05, based on a two-sided, two-sample t-test.
3.3. Laboratory Tests and Methods
Cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG) levels were measured using the homogenous colorimetric enzyme technique (Roche, Modular-P 800). Glucose was evaluated by the glucose oxidase technique (Siemens ADVIA 1800) and insulin levels were analyzed with a direct chemiluminescence technique (Siemens ADVIA Centaur, USA). Serum OPN was measured by the ELISA (enzyme-linked immunosorbent assay) method using an ELX-800 system (RayBiotech, Norcross, GA, USA) and the urinary OPN was measured using enzyme-linked immunosorbent assay by Assay Designs (Ann Arbor, Michigan, USA). Serum total cholesterol (TC) levels over 200 mg/dL, TG levels over 150 mg/dL, LDL-C levels over 130 mg/dL, or HDL-C levels under 40 mg/dL were accepted as dyslipidemia (12). IR was ascertained from the fasting plasma measurements using HOMA-IR index (insulin (mU/L) × glucose (mmol/L)/22.5) (6) and defined as a HOMA-IR index greater than 2.5 (9).
3.4. Statistical Analysis
The statistical analysis was performed using NCSS (Number Cruncher Statistical System) 2007. A t-test was used to calculate the differences between the groups. Associations were evaluated using logistic regression analysis. Categorical data were compared using the Chi-square test; P < 0.05 was accepted significant.
4. Results
This study featured 107 children (mean age 11.18 ± 3.41 years), 63 (58.9%) who were girls and 44 (41.1%) boys. Of these, 21(19.6%) were diagnosed with dyslipidemia. No significant differences in age and gender were identified between the dyslipidemic and non-dyslipidemic groups. Detailed characteristics of the selected children are shown in Table 1.
| Dyslipidemic Group (n: 21) | Non-Dyslipidemic Group (n: 86 ) | P Value | |
|---|---|---|---|
| Age, y | 10.96 ± 3.68 | 11.23 ±3.36 | 0.744 |
| Weight, kg | 67.48 ± 27.28 | 66.68 ± 24.42 | 0.896 |
| Height, m | 1.46 ± 0.21 | 1.48 ± 0.17 | 0.755 |
| BMI | 29.59 ± 5.81 | 29.19 ± 4.94 | 0.749 |
| AC | 93.61 ± 16.42 | 92.13 ± 12.78 | 0.655 |
| SBP, mmHg | 126.42 ± 19.05 | 120.57 ± 12.69 | 0.093 |
| DBP, mmHg | 67.57 ± 10.92 | 67.44 ± 8.17 | 0.954 |
| Triglycerides, mg/dL | 175.15 ± 63.47 | 97.16 ± 34.39 | 0.000 |
| Glucose, mg/dL | 92.56 ± 8.47 | 89.85 ± 8.46 | 0.192 |
| Insulin, mU/L | 28.52 ± 19.31 | 20.16 ± 15.61 | 0.039 |
| HOMA-IR | 6.75 ± 5.15 | 4.49 ± 3.83 | 0.026 |
| ALT, U/L | 34.10 ± 23.45 | 23.81 ± 10.88 | 0.004 |
| AST, U/L | 25.72 ± 9.77 | 23.13 ± 6.69 | 0.153 |
| Serum Osteopontin | 46.71 ± 21.93 | 48.71 ± 25.46 | 0.742 |
| Urine Osteopontin | 237.17 ± 140.75 | 201.93 ± 126.56 | 0.720 |
| Cholesterol, mg/dL | 181.05 ± 28.64 | 159.19 ± 23.04 | 0.000 |
| LDL, mg/dL | 105.59 ± 34.72 | 90.45 ± 23.00 | 0.017 |
Abbreviations: AC, abdominal circumference, DBP, diastolic blood pressure; SBP, systolic blood pressure.
aValues are expressed as mean ± SD.
The mean weight, height, abdominal circumference, mean systolic-diastolic blood pressure, and BMI were not found to differ between the groups. The mean TC was 181.05 ± 28.64 vs. 159.19 ± 23.04 (P = 0.001) and LDL cholesterol was 105.59 ± 34.72 vs. 90.45 ± 23.0 (P = 0.017). TG levels (175.15 ± 63.47 vs. 97.16 ± 34.39; P = 0.001) were found to be significantly higher, while HDL-C levels (45.95±15.04 vs. 53.63±16.07; P=0.05) were detected as significantly lower in the dyslipidemic group.
While the average fasting blood glucose was similar (92.56 ± 8.47 vs. 89.85 ± 8.46; P = 0.19), insulin levels were found to be higher in the dyslipidemic group (dyslipidemic 28.52 ± 19.31 vs. non-dyslipidemic 20.16 ± 15.61; P = 0.03). Among the patients with dyslipidemia, the IR ratio was 18 (85.7%).
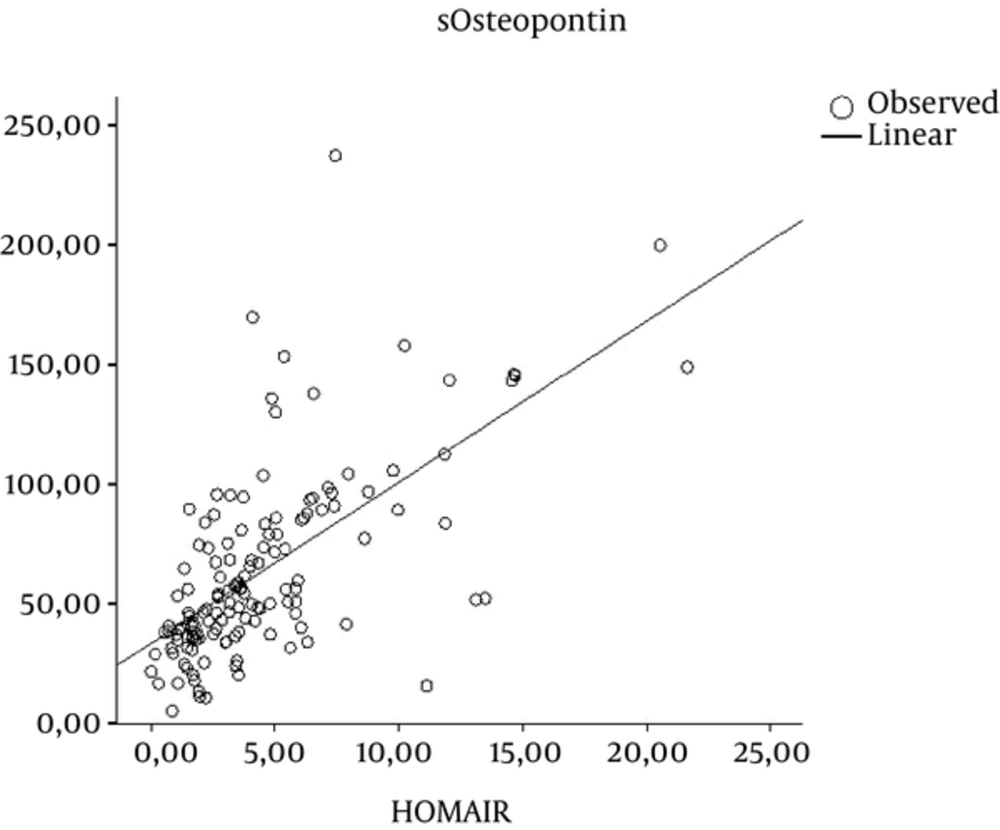
The mean serum and urinary OPN levels were not significantly higher in dyslipidemic obese children (mean serum: 46.71 ± 21.93 vs. 48.71 ± 25.46; P = 0.74; urinary OPN: 237.17 ± 140.75 vs. 201.93 ± 126.56; P = 0.30) (Figure 1). Liver function tests were evaluated for both groups and mean ALT levels (34.10 ± 23.45 vs. 23.81 ± 10.8; P = 0.04) were found to be higher in children with dyslipidemia.
In the study group, regression analysis confirmed that an increase in OPN levels is an important risk factor in IR (P < 0.001); there was no association between OPN levels and dyslipidemia in obese children (Table 2).
| Model | Unstandardized Coefficients | Standardized Coefficients | t | P Value | ||
|---|---|---|---|---|---|---|
| B | Std Error | Beta | B | Std Error | ||
| 1 | (Constant) | -2.473 | 2.562 | -0.965 | 0.337 | |
| BMI | 0.228 | 0.079 | 0.277 | 2.875 | 0.005 | |
| sOsteopontin | 0.009 | 0.016 | 0.054 | 0.570 | 0.570 | |
| uOsteopontin | 0.001 | 0.003 | 0.042 | 0.443 | 0.659 | |
5. Discussion
Obesity has become one of the foremost public health issues globally (13-15). The prevalence of the co-morbidities related to obesity has also increased with the increasing obesity prevalence (16); therefore, health-care suppliers should identify overweight and obese children for proper and early treatment. Co-morbidities, such as type 2 diabetes mellitus and steatohepatitis, previously considered as “adult diseases,” are now frequently seen in obese children. Obesity during adolescence, independent of obesity in adulthood, increases the risk of disease and premature death during adulthood (17-20). “Metabolic syndrome” is a term used to describe the clustering of metabolic risk factors for type 2 diabetes and atherosclerotic cardiovascular disease in adults: abdominal obesity, hyperglycemia, dyslipidemia, and hypertension. Several studies have estimated that approximately 10% of US adolescents have metabolic syndrome, as defined by modifications of adult criteria (21-23). Dyslipidemia occurs amongst overweight and obese children and adolescents, particularly those with a central fat distribution and increased adiposity (as measured by triceps skin-fold thickness ≥ 85th percentile). The typical pattern features raised concentrations of blood serum, LDL cholesterol, and TG and also reduced concentration of HDL cholesterol (24, 25).
The risk of these abnormalities increases with the severity of obesity (26). In our study, the mean age for obesity was found to be 11.18 years and the dyslipidemia ratio was 19.6% in these children. The mean cholesterol, LDL-C, and TG levels were higher, while the HDL-C level was lower in the dyslipidemic group.
In a study involving more than 6,000 sixth grade students (average age 11.8 years), nearly 20% were overweight and 30% were obese. Impaired fasting glucose (FPG ≥ 100 mg/dL) was present in 15.5% of overweight children, 20.2% of obese children, and 22.5% of severely obese children (27, 28).
The present study did not detect any difference in mean fasting blood glucose levels between the two groups, however, insulin levels were higher in the dyslipidemic group. High IR detected in the dyslipidemic group was not found to be significant.
OPN has been established as a major component in the development of adipose tissue inflammation and IR, and some human studies have focused on its role in patients with obesity. OPN expression in adipose tissue, as well as in circulating OPN levels, was substantially elevated in obese patients compared with lean subjects and was further increased in obese diabetic or insulin-resistant patients (29, 30). However, in our study, serum OPN levels were not found to be higher in the dyslipidemic group.
OPN is a secretory protein that also plays a significant role in urinary stone formation. In our study, no significant difference was detected in urinary OPN levels in dyslipidemic children.
Obesity is associated with a clinical spectrum of liver abnormalities collectively known as nonalcoholic fatty liver disease (NAFLD), the foremost reason for liver disease in childhood (31, 32). There are vital clinical associations between NAFLD and parts of the metabolic syndrome, as well as IR, dyslipidemia, and high blood pressure, regardless of the degree of obesity (33). We also found lower mean ALT levels (34.10 ± 23.45 vs. 23.81 ± 10.8; P = 0.04) in non-dyslipidemic children. With the increased prevalence of childhood obesity, NAFLD is increasingly seen in children (34). Previous studies have reported that the incidence of hepatosteatosis in obese children was 12 to 72.9% (27-29). Hepatosteatosis was detected by abdominal ultrasonography in 61.7% of obese children involved in our study.
In conclusion, while our findings confirmed the correlation with serum osteopontin levels and insulin resistance, increased osteopontin was not found related to dyslipidemia.