1. Background
Kawasaki disease (KD) is characterized by systemic inflammation in medium-sized arteries and especially, predominantly affects children under 5 years of age (1). Like that although many clinical features such as aseptic meningitis that is not typically included in the diagnostic criteria for KD it is known to occur with KD, arthritis is also accompanied with KD but is not concerned recently. However, arthritis is a common component of KD and has been divided into early-onset (39%) and late-onset arthritis (61%) (1, 2). Early onset type in KD occurs during the first 7 - 10 days of illness, continues for 20 days, and tends to involve multiple joints such as small interphalangeal joints as well as large weight-bearing joints (1, 2). On the other hand, late-onset type develops usually on the 10th day of illness or later, generally affects especially the knees and ankles, and lasts in many different days from 15 days to 6 - 8 weeks (1-4). In general, arthritis in KD reacts dramatically when the treatment with intravenous immunoglobulin (IVIG) is started and is a non-erosive synovitis that has excellent outcomes with no sequelae (1, 3, 4). Arthritis was well documented in KD patients in the pre-IVIG era, but it was recently reported that about 2% of KD patients experienced arthritis in the defervescent state following IVIG management and needed corticosteroid treatment or a second use of IVIG (3, 5, 6).
2. Objectives
We aimed to report the prevalence and clinical course of arthritis in KD as well as to establish its relationship to coronary outcomes. In addition, we aimed to compare the clinical manifestations, laboratory findings, and presence of chronic arthritis between patients with early- and late-onset arthritis.
3. Methods
The Institutional Review of Board of Sungkyunkwan University Health System approved this retrospective study (IRB 2016-12-009). We retrospectively reviewed the medical records of patients who were treated by admitting to Samsung Changwon Hospital with a diagnosis of KD between March 2006 and February 2016. According to the 2017 American Heart Association (AHA) guidelines for KD, a diagnosis of complete KD (cKD) was made on the clinical criteria in addition to the exclusion of alternative diagnoses (1). Incomplete KD (iKD) was diagnosed in any infant or child with prolonged unexplained fever, fewer than 4 of the principal clinical findings, and compatible laboratory or echocardiographic findings (1). Echocardiogram was considered positive if any of 3 conditions are met: Z score of left anterior descending coronary artery or right coronary artery ≥ 2.5; coronary artery aneurysm is observed; or ≥ 3 other suggestive features exist, including decreased left ventricular function, mitral regurgitation, pericardial effusion, or Z scores in left anterior descending coronary artery or right coronary artery between 2 and 2.5 (1). In this study we defined arthritis as the presence of pain, heatness regardless of redness or swelling at specific joints. Swelling or redness of the extremities were included in the clinical symptoms for KD, therefore this symptom was not included in the definition of arthritis in this study. Moreover, because the redness and swelling of arthritis is localized symptom, it can differentiate from its as the symptom of the KD. Also we divided arthritis in KD into early-onset type, which developed during the first 7 - 10 days of illness and late-onset type, which developed on the 10th day of illness or later according to the definition of previously reported literatures (1-5). Clinical characteristics and laboratory results, response to IVIG, arthritis onset time, number and sites of affected joints, length of hospital stay, and coronary artery outcomes were reviewed. We also described the clinical characteristics, coronary artery change between patients with only arthralgia and patients with all type arthritis.
3.1. Statistical Analysis
All statistical analyses were two-sided, and P values < 0.05 were considered statistically significant and all parameters were expressed as mean ± standard deviation. An independent t-test and Pearson’s chi-square test was used to compare the statistical difference between early and late onset type arthritis patients. IBM SPSS V. 21.0 software (IBM Inc., Chicago, IL, USA) was used for statistical analysis.
4. Results
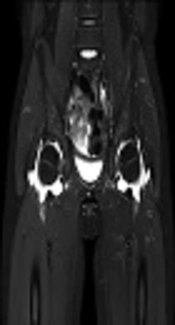
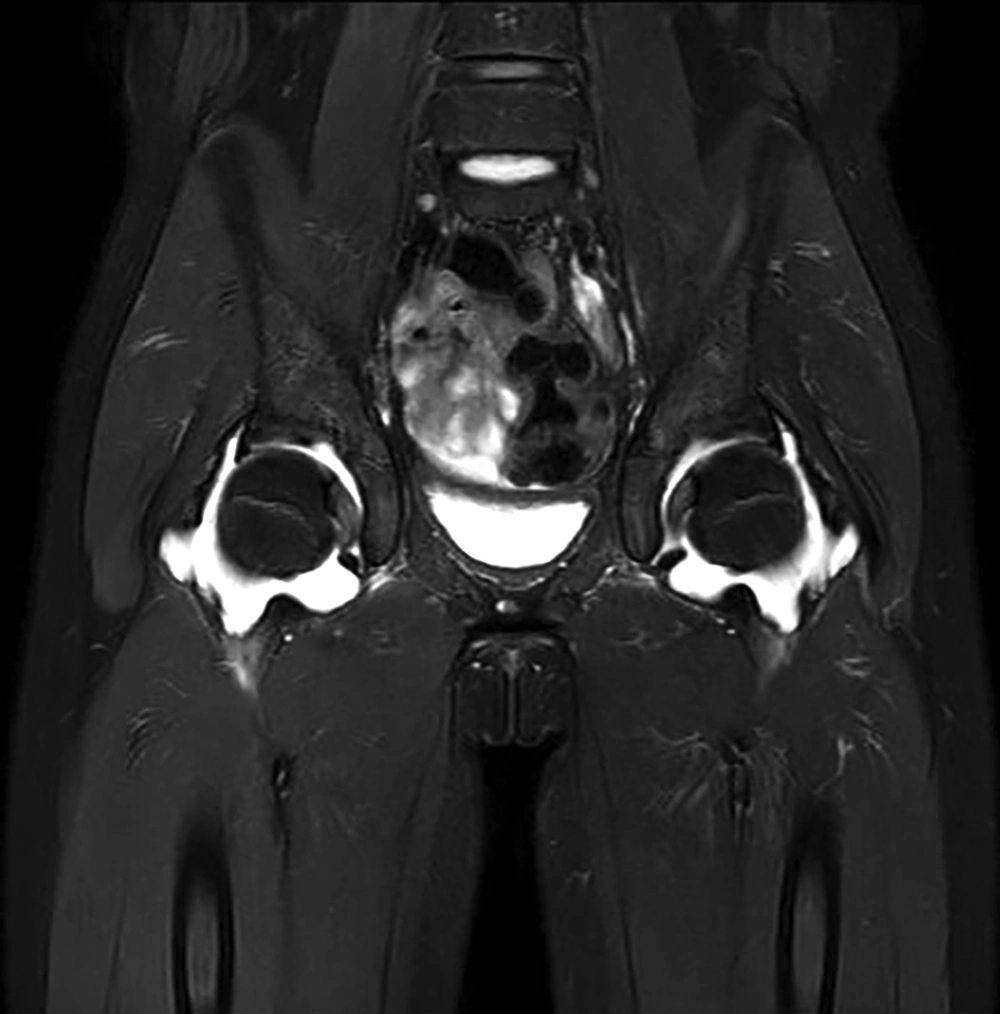
The 547 children were diagnosed with KD between March 2006 and February 2016. After excluding 23 patients who were lost to follow-up, a total of 524 patients from this 10-year period were included in this study. Among them, 189 (36.1%) were diagnosed with iKD. The overall prevalence of arthritis (including both early- and late onset) with KD was 17.6 % (n = 92/524). Table 1 describes the characteristics of patients with KD arthritis. Patients with late-onset arthritis were older and had a longer fever duration, more frequent edema in the extremities, higher neutrophil counts, and higher levels of N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP). There were no statistical differences in gender, conjunctival injection, oral mucosa changes, cervical lymphadenopathy, polymorphous rashes, and other markers of inflammation such as white blood cell (WBC) counts, erythrocyte sedimentation rate (ESR), or c-reactive protein (CRP). There were also no differences in the response to IVIG therapy, and a second dose of IVIG was required in 5 and 3 cases of early- and late-onset arthritis, respectively. The use of methylprednisolone or humanized monoclonal antibody against tumor necrosis factor-α (TNF-α) was not found in this study. Table 2 shows coronary artery lesions (CALs) including ectasia and aneurysm were not different between groups of early- and late-onset arthritis. Also when we analyzed the coronary outcomes between subjects with and without arthritis, there was no statistical significance (Table 3). Table 4 describes the orthopedic characteristics of patients with arthritis. Patients had similar proportions of oligoarticular involvement (71.8 vs. 66.7%), with the knee joint predominantly affected (90.1 vs. 95.2%) regardless of early- vs. late-onset arthritis, respectively. Then, hand, hip and ankle joints were affected in order regardless of arthritis type. Table 5 also shows the orthopedic characteristics of patients according to the type of Kawasaki disease. Our study showed that hip joint was more predominantly involved in the complete Kawasaki disease, whereas wrist and metacarpophalangeal joints of fingers were more predominantly involved in the incomplete Kawasaki disease. 17 (23.9%) and 6 (28.6%) patients with early- and late-onset arthritis had simple X-rays done by pediatric orthopedic surgeons and among them, 2 (2.8%) and 2 (9.5%) patients had ultrasonography, respectively and there were fluid collections within joint cavity. All most of patients with arthritis related to KD were treated with supportive care. In our sample, only one patient with late-onset arthritis underwent magnetic resonance imaging (MRI) and a diagnostic arthrotomy of the hip because of severe pain and moderate fluid collection seen on MRI with regard to rule out septic arthritis (Figure 1). There was infiltration of leukocytes but no hypertrophy of the synovium. Also this synovial fluid’s Gram and Wright’s staining revealed no bacteria. The arthritis did not progress to pannus formation and joint destruction. MRI findings improved within 3 months after onset in this patient.
| Variables | Early Onset (N = 71) | Late Onset (N = 21) | P Value |
|---|---|---|---|
| Age, months | 32.7 ± 12.1 | 62.5 ± 23.8 | 0.032b |
| Male/Female | 35/36 | 13/8 | 0.697 |
| Fever duration, days | 5.8 ± 1.94 | 7.11 ± 2.51 | 0.020b |
| Conjunctival injection, % | 92.9 | 100 | 0.807 |
| Oral mucosa change, % | 80.3 | 94.7 | 0.468 |
| Cervical lymphadenopathy, % | 54.7 | 35.5 | 0.483 |
| Swelling or redness of extremities, % | 34.1 | 72.3 | 0.027b |
| Polymorphous rash, % | 83.1 | 78.9 | 0.591 |
| WBC count, /μL | 13800 ± 4200 | 14900 ± 5087 | 0.804 |
| Neutrophil, % | 58.69 ± 13.84 | 72.16 ± 16.43 | 0.009b |
| Platelet, × 103/μL | 370.41 ± 117.69 | 315.94 ± 85.71 | 0.352 |
| ESR, mL/h | 34.45 ± 23.87 | 41.35 ± 32.76 | 0.452 |
| CRP, mg/L | 64 ± 46.4 | 94.8 ± 76.48 | 0.059 |
| ProBNP, pg/mL | 1343.80 ± 1080.44 | 3754.42 ± 4272.71 | 0.008b |
| Na, mmol/L | 136.25 ± 2.54 | 136.2 ± 3.13 | 0.524 |
| Albumin, g/dL | 3.5 ± 0.4 | 3.3 ± 0.58 | 0.891 |
| AST, IU/L | 58 ± 54.4 | 57.7 ± 43.05 | 0.723 |
| ALT, IU/L | 71.7 ± 84.2 | 74.1 ± 79.28 | 0.428 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ProBNP, prohormone brain-type natriuretic peptide; WBC, white blood cell.
aValues are shown as mean ± SD.
bP value < 0.05 was statistically significant.
| Variables | Early Onset (N = 71) | Late Onset (N = 21) | P Value |
|---|---|---|---|
| Normal | 59 (83.1) | 16 (76.2) | 0.168 |
| Ectasia | 7 (9.9) | 3 (14.3) | 0.071 |
| Aneurysms | 5 (7.0) | 2 (9.5) | 0.217 |
| Small (< 5 mm) | 3 (4.2) | 1 (4.75) | 0.523 |
| Medium (5 ~ 8 mm) | 1 (1.4) | 1 (4.75) | 0.143 |
| Large (> 8 mm) | 1 (1.4) | 0 (0) | 0.782 |
aValues are expressed as No. (%).
| Variables | Non-Arthritis (N = 432) | Arthritis (N = 92) | P Value |
|---|---|---|---|
| Normal | 396 (91.7) | 75 (81.5) | 0.067 |
| Ectasia | 21 (4.9) | 10 (10.9) | 0.052 |
| Aneurysms | 15 (3.5) | 7 (7.6) | 0.571 |
| Small (< 5 mm) | 11 (2.5) | 4 (4.4) | 0.457 |
| Medium (5 ~ 8 mm) | 2 (0.5) | 2 (2.2) | 0.079 |
| Large (> 8 mm) | 2 (0.5) | 1 (1.1) | 0.142 |
aValues are expressed as No. (%).
aValues are expressed as No. (%).
bP value < 0.05 was statistically significant.
cJoints including wrist and metacarpophalangeal joints of fingers.
Abbreviations: cKD, complete Kawasaki disease; iKD, incomplete Kawasaki disease.
aValues are expressed as No. (%).
bP value < 0.05 was statistically significant.
cJoints including wrist and metacarpophalangeal joints of fingers.
5. Discussion
The main findings of this study showed that late-onset arthritis in KD was found in older patients with a longer duration of fever, more frequent edema in the extremities, higher neutrophil counts, and higher levels of NT-proBNP compared to early-onset arthritis patients. However, there was no relationship with coronary artery lesions (CALs) and type of arthritis in KD.
KD is a multi-systemic vasculitis, which is characterized by systemic inflammation in medium-sized arteries and especially, predominantly affects children under 5 years (1). It can accompany with several complications such as aseptic meningitis, anterior uveitis, gallbladder hydrops, cranial nerve palsies, ischemic colitis, pancreatitis, and arthritis or arthralgia during the natural course (1). Arthritis and arthralgia are not included in the diagnostic criteria of KD but usually observed in the acute or subacute stage in 15% ~ 45% of KD patients (1, 3). Arthritis in KD has been divided into two types; early onset arthritis, which develops during the first 7 - 10 days of illness, continues usually for 20 days, and tends to involve multiple joints (polyarticular). It is often impossible to distinguish from systemic juvenile idiopathic arthritis (JIA) (2, 3). However, late-onset arthritis, which develops on the 10th day or later of illness, occurs more often in large weight bearing joints (usually pauciarticular), especially the knees and ankles, and lasts for 15 days to 6 - 8 weeks (1-4). However, it was recently reported that about 2% of KD patients experienced arthritis in the defervescent state following IVIG management, requiring corticosteroids or a second course of IVIG (3, 5, 6). In addition, from before IVIG was established as a gold standard treatment for KD, it was reported that early-onset arthritis was associated with poor cardiac outcomes such as coronary artery aneurysms (3). Therefore, we wondered whether there would be a clinical and cardiac difference between early- and late-onset arthritis in KD patients. In this study, while the overall prevalence of arthritis (including early- and late onset) in KD was 17.6 %, previously reported prevalence was 7.5% ~ 31% (3). We found that patients with late-onset arthritis were older and had longer fever durations, more frequent edema in the extremities, lower neutrophil counts, and higher levels of NT-proBNP. Current report suggests that NT-proBNP has high diagnostic value for identifying or distinguishing KD in the children with undifferentiated febrile illness (7, 8), and may be effective predictor for IVIG resistance and develop of CALs in KD patients (9). However, in our study, although patients with late onset arthritis had higher levels of NT-proBNP than subjects with early onset arthritis, there was no correlation with CALs between two groups. And changes in oral mucosa, conjunctival injections, cervical lymphadenopathy, rashes, and other markers of inflammation, including WBC, ESR and CRP, were not significantly different in children with early- vs. late-onset arthritis. There were also no differences in the response to IVIG therapy in this study. We think this finding may be related to the natural self-recovered course and good prognosis of late onset arthritis. After discharge, when the patients with KD visited the outpatient clinic, there might be no complaints related to arthritis because patients had already self-recovered during home staying. In this point, we hypothesize that late onset arthritis with KD resembles their clinical character with reactive arthritis. Reactive or post-infectious arthritis is an inflammatory, asymmetric, aseptic arthritis associated with certain infections, particularly in susceptible individuals for Streptococcus, Chlamydia, Salmonella, Shigella, Campylobacter, and Yersinia (6, 10, 11). Reactive arthritis develops within 1 ~ 4 weeks after the primary infection. Although infection plays a major role in its etiology, it is removed from the site of primary infection and is distinguished from septic arthritis by lack of organisms in the involved joint and inflammatory rather than infectious findings on synovial fluid analysis (11). We suggest that late onset arthritis with KD is a reactive arthritis due to an unknown pathogen according to the fact that the synovial finding resembles that of reactive arthritis, it occurs after high dose IVIG therapy, and it develops regardless of taking high-dose acetylsalicylic acid every 6 hours, with a total daily dose of 50 to 100 mg/kg/d in the subacute stage of KD (1, 6, 12). Unlike the literature that early-onset arthritis was associated with poor cardiac outcomes such as coronary artery aneurysms (3), late onset arthritis may be mild form reactive arthritis without coronary artery changes regardless of being older and having longer fever durations, more frequent edema in the extremities, lower neutrophil counts, and high level of NT-proBNP according to our study results.
Although the mechanisms of arthritis in KD have not been obviously understood, arthritis is considered to reveal a local inflammation of joints triggered by the inflammatory cascade in KD. An inflammatory phenomenon which noted in the synovial fluid of KD supports the inflammatory nature of KD arthritis (3). Inflammatory cytokines, such as interleukin (IL)-1, IL-2, IL-6, IL-8, interferon-γ, and TNF-α, have been demonstrated to be elevated in KD, and they are mediators the clinical and laboratory features of acute phase in KD (13-15). In addition, serum matrix metalloproteinase-3 (MMP-3), which is associated with the destruction of cartilage and remodeling of connective tissues such as synovium and vascular walls, is increased in the acute phase and the level of MMP-3 is higher at diagnosis than at arthritis remission (4, 16). Therefore, it appears that inflammatory reaction of arthritis develops during the acute stage of KD and clinical symptoms become apparent after defervescent state (4). Synovial enhancement on MRI observed in KD arthritis reflects hyperemia and inflammation of the synovium, which is also commonly observed in rheumatoid arthritis (RA) and JIA (4). However, in contrast to RA or JIA, there were no bony erosion, irregular lining of the synovium, or bone marrow edema and did not progress to form a pannus and resultant joint destruction in KD (4). And also MRI findings in our case improved within 3 months after onset like the literature, suggesting that arthritis in KD may be self-limited (4). Unlike the cases described in the literature, our patient did not have a second episode of arthritis (5). In this mention, when there is an onset of arthritis related to KD, it is not necessary to perform more aggressive diagnostic procedures such as MRI or diagnostic arthrotomy.
5.1. Limitations
This study has some limitations. Our data were limited to a single hospital’s experience and this study had a retrospective design with a low number of patients. Therefore, these results may not be generalizable to the overall pediatric population. Selection bias related to age may influence study results. We think that KD patients might experience arthritis more frequently because when considering the pathogenesis of KD as a systemic vasculitis, the swelling and tenderness of extremities may be combined with arthritis, but could not complain because KD is more frequent in young children. In this study, the youngest child who complained of arthritis was 17 months old. However, this study demonstrates that arthritis in KD is a benign and self-limited without sequelae in most patients regardless of onset time.
5.2. Conclusions
Although late-onset arthritis was found in older patients with a longer duration of fever, more frequent edema in the extremities, higher neutrophil counts, and higher levels of NT-proBNP compared to early-onset arthritis patients, there was no association with CALs and type of arthritis in KD. Arthritis associated with KD was usually involving the knee joints with excellent outcomes.