1. Background
Inhaled corticosteroids (ICSs) are the most effective and most commonly used treatment for asthma (1, 2). ICSs have been shown to effectively reduce airway inflammation, control asthma symptoms, improve the quality of life, improve the lung function, reduce airway hyperresponsiveness, alleviate the frequency and severity of attacks, and reduce mortality due to asthma (1, 2). Hypothalamic-pituitary-adrenal (HPA) axis suppression is the most important systemic side effect associated with ICS therapy, and its mechanism is well described. The incidence of the side effects increases parallel to the increase in the daily doses (2, 3). Chronic exposure and high doses might result in HPA axis deficiency, adrenal gland atrophy, and insufficient cortisol production due to adrenal suppression. Patients might present with adrenal failure, as well as non-specific symptoms such as malaise, headache or abdominal pain or more serious symptoms, such as growth retardation, syncope, hypotension, hyponatremia or hypoglycemia (4, 5).
2. Objectives
This study aims to evaluate the prevalence and risk factors of HPA axis suppression in children aged 6 to 18 years followed-up with the diagnosis of persistent asthma and have been under regular ICS therapy for a minimum of 3 months.
3. Methods
3.1. Study Design and Settings
The present cross-sectional study was conducted at Sisli Hamidiye Etfal Research and Training Hospital, between June 2015 and December 2015. Informed consent was obtained from the subjects and their guardians. The parents and patients were informed about the test to be performed by the same physician.
3.2. Study Population
The subject group included pediatric patients (6- to 18-years-old), who were being followed-up with the diagnosis of persistent asthma and had been receiving regular inhaled corticosteroid therapy for at least 3 months. Those who had received systemic corticosteroid therapy within the last 3 months, who had intermittent asthma, received irregular inhalation therapy or had missing information in their files, were excluded from the study.
3.3. Study Protocol
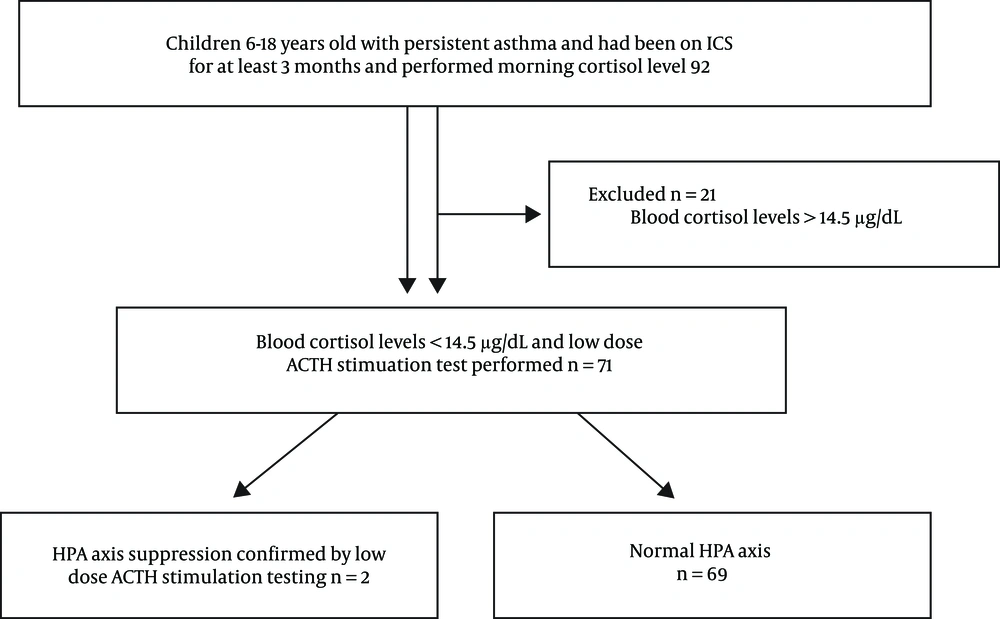
The flowchart of the study is presented in Figure 1. All of the subjects were evaluated for symptoms and signs of adrenal failure. We asked the enrollees about inappetence or increased appetite, nausea, malaise, morning headaches, failure to gain weight associated with HPA axis suppression; autonomic symptoms pointing to hypoglycemia; abdominal pain, fatigue, weakness, myalgia, growth retardation and other subjective clinical signs associated with suppression. Patients with a minimum of three symptoms were included in the study.
Data were recorded for each patient, including the age, gender, height, weight, age at diagnosis, hospitalization in the last one-year period, ICS therapy (type, dose, duration), and other therapies (long-acting β2-agonists or nasal corticosteroids) received. The severity of the asthma was classified as mild, moderate or severe persistent asthma according to the National Asthma Education and Prevention Program (NAEPP) guideline (6). Patients with mild asthma were treated with low ICS daily dose, moderate asthma with medium daily dose and severe asthma with medium to high daily dose as per NAEPP guideline (6). ICS was given as metered dose inhaler (MDI), whereas ICS + LABA were given as dry powder inhaler to 12 years and older. Total IgE (T. IgE), inhalant panel, allergy skin test, and pulmonary function test (PFT) were obtained from the patient files. The forced expiratory volume at 1 second to forced vital capacity (FEV1/FVC) ratio lower than 80% were considered as a bronchoconstrictive change (7).
3.4. Cortisol Test
The metyrapone and insulin tolerance tests are considered the gold standards to evaluate HPA axis function in asthmatic patients (8). In the literature, studies conducted using the metyrapone test have revealed the prevalence of HPA axis dysfunction as 65% and HPA axis suppression as 16% (9). These rates are suggested to be even higher in asthma patients who receive concomitant ICS and nasal steroid (NS) therapy (10). However, since metyrapone is not licensed in some countries, including Turkey, testing is limited. On the other hand, the insulin tolerance test is not preferred as its safety in children has not been established (4).
The ACTH stimulation test has two variants, the standard (250 μg) and low-dose (1 μg) options (4). The low-dose ACTH stimulation uses a physiological dose and is a safe and convenient method (11). Moreover, compared to the standard-dose ACTH test, the low-dose ACTH stimulation is a more reliable test, with 100% specificity and 89% sensitivity (12, 13). The study subjects were referred to the Pediatric Endocrine Outpatient Clinic. After being evaluated by a pediatric endocrine specialist, subjects were scanned for baseline fasting blood cortisol levels at 08:00 am (14, 15). Patients whose cortisol levels were lower than 14.5 μg/dL (400 nmol/L) were given the low-dose ACTH stimulation test between 08:00 and 10:00 am. After the patients’ baseline blood samples were collected, 1μg of synthetic ACTH (Synacthen® 0.25 mg, Novartis, Germany) was administered intravenously after reconstitution with 0.9% saline. The drug was procured from abroad. After the administration of the ACTH, to measure the serum cortisol response, blood samples were collected after 30 and 40 min. HPA axis function was considered normal in patients with any cortisol level above 18 μg/dL (500 nmol/L) (4, 16, 17). The demographic characteristics of the patients, the clinical and laboratory features of asthma, ICS dose, duration of treatment, and the LABA and nasal steroid use were compared between the groups of patients with normal HPA function and those with suppressed HPA axis, and the risk factors for these characteristics were analyzed.
3.5. Statistical Analysis
Statistical analyses were performed using the SPSS 15.0 for Windows software. For the numerical variables, descriptive statistics were given as the mean, standard deviation, minimum, and maximum. Since the condition for normal distribution was not fulfilled, the numerical variables between the two independent groups were compared using the Mann Whitney U-test. The ratios between the groups were compared with the help of the chi-square test. The Monte Carlo simulation was used wherever the conditions were not fulfilled. The alpha statistical level of significance was based on a value of P < 0.05.
4. Results
Ninety-two patients with persistent asthma, among which 51 (55.4%) were male, were included in the study. HPA axis suppression was observed in 2 (2.1%) of the 92 patients with low morning serum cortisol level. The demographic characteristics of the patients with and without HPA axis suppression are presented in Table 1. No statistically significant difference in terms of age, gender, weight, and height were observed between the groups (P > 0.05). The BMI values of the patients with HPA axis suppression, as measured by the low-dose ACTH test, were significantly lower than those without HPA axis suppression (P = 0.034).
| Normal HPA Axis, N = 69 (92.2%) | HPA Axis Suppression, N = 2, (2.8%) | P Value | |
|---|---|---|---|
| Age | 10.2 ± 2.6 | 8.5 ± 0.7 | 0.342 |
| Gender | 1.000 | ||
| Female | 32 (46.4) | 1 (50.0) | |
| Male | 37 (53.6) | 1 (50.0) | |
| Weight, kg | 43.3 ± 14.8 | 30.3 ± 8.1 | 0.144 |
| Height, cm | 142.1 ± 16.4 | 140.0 ± 14.1 | 0.903 |
| BMIb | 21.0 ± 4.2 | 15.3 ± 1.1 | 0.034 |
| BMI | |||
| Normal | 30 (43.5) | 2 (100) | 0.695 |
| Underweight | 1 (1.4) | 0 (0.0) | |
| Overweight | 14 (20.3) | 0 (0.0) | |
| Obese | 24 (34.8) | 0 (0.0) |
Abbreviation: BMI, body mass index
aValues are expressed as mean ± SD or No. (%).
bMann Whitney U test; chi square
The clinical features of asthma and the laboratory test results of the patients with and without HPA axis suppression, as measured by the low-dose ACTH test, are shown in Table 2. Both groups were comparable in terms of the age at the diagnosis of asthma, the severity of asthma, and the number of hospitalizations due to acute asthma attacks in the last 1-year period. No statistically significant difference was observed in terms of the laboratory features of asthma including the T. IgE levels, elevated age-related T. IgE, positive inhalant panel, positive skin test, and PFT (P > 0.05). When the characteristics of the asthma treatment were compared between the patients with and without HPA axis suppression, as measured by the low-dose ACTH test, no statistically significant difference was found in terms of the nasal steroid use, duration of ICS therapy, ICS dose, ICS type, cumulative dose or blood cortisol level (P > 0.05).
| Normal ACTH Axis, N = 69 (92.2%) | ACTH Axis Suppression, N = 2 (2.8%) | P Valueb | |
|---|---|---|---|
| Age at diagnosis of asthma | 6.3 ± 3.5 | 5.0 ± 1.4 | 0.565 |
| Asthma severity | |||
| Mild | 33 (47.8) | 0 (0.0) | 0.526 |
| Moderate | 34 (49.3) | 2(100) | |
| Severe | 2 (2.9) | 0 (0.0) | |
| Hospital admission due to asthma in the last 12 months | 21 (30.4) | 0 (0.0) | 1.000 |
| T. IgE levels | 481.0 ± 609.7 | 607.0 ± 834.4 | 0.941 |
| T. IgE (high) | 43 (66.2) | 1 (50.0) | 1.000 |
| Inhalant panel (positive) | 38 (63.3) | 1 (50.0) | 1.000 |
| Skin test (positive) | 26 (66.7) | 1 (100) | 1.000 |
| PFT (obstructive changes) | 13 (18.8) | 0 (0.0) | 1.000 |
| Nasal steroid | 45 (65.2) | 2 (100) | 0.546 |
| ICS duration, mo | 11.1 ± .8 | 9.0 ± 0.0 | 0.643 |
| ICS dose, µg/day | 468.0 ± 122.8 | 500.0 ± 0.0 | 0.609 |
| ICS type | |||
| ICS | 50 (72.5) | 2 (100) | 1.000 |
| ICS + LABA | 19 (27.5) | 0 (0.0) | |
| Blood cortisol levels, mean ± SD/min-max | 9.2 2.7 | 7.9 ± 7.0 | 0.879 |
| Cumulative dose, mg | 46.3 ± 140.7 | 121.5 ± 6.4 | 0.821 |
Abbreviations: ICS, inhaled corticosteroids; LABA, long acting β2 agonist; PFT, pulmonary function test
aValues are expressed as mean ± SD or No. (%).
bMann Whitney U test, chi square test
The clinical characteristics of the two patients with HPA axis suppression, as measured by the low-dose ACTH test, are shown in Table 3.
| Case 1 | Case 2 | |
|---|---|---|
| Age, y | 9 | 8 |
| Gender | K | E |
| Asthma severity | Moderate persistent | Moderate persistent |
| BMI, kg/m2 | 16 | 14.5 |
| T. IgE, high | no | yes |
| Inhalant panel | negative | positive |
| Skin test | negative | positive |
| PFT | normal | normal |
| ICS dose, µ/day | 500 | 500 |
| ICS duration, months | 9 | 9 |
| Blood cortisol levels, nmol/L | 12.8 | 2.9 |
| ICS | Fluticasone propionate | Fluticasone propionate |
| Nasal steroid | yes | yes |
Abbreviations: BMI, body mass index; ICS, inhaled corticosteroids; PFT, pulmonary function test
5. Discussion
In this study, we have examined the prevalence of HPA axis suppression as measured by the low-dose ACTH stimulation test in children aged 6 to 18 years, followed-up with the diagnosis of persistent asthma, and receiving regular ICS therapy for a minimum of 3 months. According to our results, the rate of the HPA axis suppression is 2.1%. This ratio is considerably lower than the 7.7% - 65.1% prevalence reported in the literature, although this varies depending on the test method (7, 9, 12, 17-21). When we compared the patients with and without HPA axis suppression in terms of the demographics and the laboratory and clinical characteristics, we observed no statistically significant differences between the groups, except for the BMI values. However, it should be noted that our statistical comparison is based on only two patients, in which we have observed HPA axis suppression. In a similar study conducted at the Hacettepe Faculty of Medicine by Cavkaytar et al. (18), the prevalence of the HPA axis suppression was 7.7%. This study focused on the HPA axis suppression rate and had retrospectively evaluated 91 patients with a reported treatment compliance of 80% to 90%, which might explain the higher prevalence observed. In our opinion, since our subject group comprised patients from lower socioeconomic groups presenting to our hospital, the lower ratio of HPA axis suppression we observed might be associated with an inaccurate inhaler technique, popular misconceptions about the therapy among patients (e.g., that the drugs cause short stature, trigger asthma), and least likely noncompliance since the drug use was queried by two physicians. Patients who had received systemic steroid therapy due to asthma attacks or for asthma control within the last 3 months were excluded from the study. On the other hand, the study by Cavkaytar et al. (18) included patients who had received systemic steroids due to asthma attacks within the last 3 months and the rate of systemic steroid use due to asthma attacks was higher in the group with HPA axis suppression. A large-scale, case-control study conducted by Mortimer et al. (22) has shown that exposure to oral corticosteroids increases the risk of adrenal failure in patients treated with ICSs. We believe that the exclusion of the patients treated with systemic steroids from our study was a factor leading to lower rates of HPA axis suppression. The best test for evaluating HPA axis function is a controversial topic. In the study conducted by Cavkaytar et al. (18), similarly to our study, the low-dose ACTH test was performed in patients with low morning cortisol levels. However, these authors have determined the limit for peak cortisol level as 19.8 μg/dL. In our study, the limit for peak cortisol level was 18 µg/dL. In the literature, peak cortisol levels < 18 μg/dL are reported to have 90% sensitivity and specificity in the diagnosis of adrenal suppression (4). We have chosen our limit based on this finding.
There are studies suggesting that long-term nasal steroid therapy used in the treatment of allergic rhinitis might increase HPA axis suppression when combined with ICSs (11, 12). While the ICSs enter the pulmonary circulation after being absorbed from the lungs, nasal steroids directly enter the systemic circulation through the nasal mucosa and lead to stronger systemic effects than the ICSs. This might explain the suppressive effects of nasal steroids. A study conducted by Zollner et al. (10) reported an HPA axis suppression rate of 35% and suggested that the use of nasal steroids increases the risk of HPA axis suppression. In another study conducted by Zollner et al. (9), a significant correlation was observed between nasal steroids and HPA axis suppression. Also, in our study, the two patients with HPA axis suppression were using nasal steroid therapy.
In the literature, low BMI values are reported among the risk factors of adrenal suppression (14). In our study, the BMI values of the patients who developed HPA suppression were significantly lower than the group with normal HPA axis although this finding depends on only 2 patients. In a study conducted by Zollner et al. (9), the prevalence of HPA axis suppression was lower among patients with higher BMI values. Thus, higher BMI is thought to have a protective effect against HPA axis suppression.
Adrenal failure is one of the acute and life-threatening emergencies faced by physicians. In the literature, there are case reports on adrenal crises that developed secondary to ICS therapy (23, 24). In a case series published by Patel et al. (24) comprising eight patients who developed symptomatic adrenal failure due to ICS therapy, two patients were admitted with acute conditions due to hypoglycemia. Drake et al. (25) reported symptomatic hypoglycemia without cushingoid appearance due to adrenal failure in four patients who were receiving high-dose fluticasone propionate therapy (> 500 μg/day). In a survey study from the literature, among 33 patients (28 children, 5 adults) admitted with an acute adrenal crisis, of which 23 were comatose and had convulsions due to acute hypoglycemia, 11% could not be diagnosed at the first presentation (23). Therefore, pediatricians should be vigilant in case of children with asthma receiving ICS therapy and consider adrenal failure in patients with impaired consciousness, abnormal behavior or autonomic symptoms suggesting hypoglycemia. In our study, none of the patients with HPA axis suppression developed cushingoid appearance or an adrenal crisis. With its many proven benefits on morbidity and mortality in children and adults, ICS therapy forms the basis of asthma therapy and continues to play a critical role in the treatment of childhood asthma. Although systemic side effects due to ICS (e.g., adrenal suppression) usually occur at high doses and through long-term therapy, such effects have also been reported, though rarely, with low- to moderate-dose ICS during short-term treatment (5). Increased awareness, early identification of patients under risk, regular follow-up of the patients to determine the minimum effective ICS dose to control the condition, and reduction of the dose with disease control, are required to reduce the risk of adrenal suppression. Also, selecting the ICS therapy that will cause minimum systemic effects and revision of the therapy before increasing the dose in children with a low level of response might also be helpful (4, 20).
In conclusion, we have observed a 2.1% prevalence of HPA axis suppression in children with asthma who were being followed-up with the diagnosis of persistent asthma and receiving regular ICS therapy. All children in the present study were on medium to low dose of ICS. Children with persistent asthma who has been treated with regular ICS should be screened for HPA axis suppression.
The limitations of our study include the low number of subjects, low sensitivity of the measurement of the morning cortisol levels and the inability of normal values to rule out HPA axis suppression, low number of patients with severe asthma, as well as the unavailability of metyrapone, which has been established as the gold standard dynamic test to evaluate HPA axis function, in our country. Another limitation is the study design in which the cross-sectional study does not allow for follow up or repeating the tests.