1. Background
Isoflurane, with its quick induction, fast emergence and hemodynamic stability has gained widespread acceptance for use on pediatric patients. Nevertheless, Isoflurane has been considered to be connected with EA in pediatrics in early studies. Emergence agitation (EA) in the pediatric patient soon after the end of anesthesia is not uncommon, with occurrence ranging from 10 to 80 percent (1-3). EA is identified by patients’ behavior including crying, excitation, irritability, uncooperativeness, disorientation, delirium and thrashing (4-6).
EA is experienced by children undergoing various medical operations. While various factors have been considered as etiologies of EA, there is no single complete factor that can cause EA. Numerous factors have been recommended, and many factors, such as preschool age, pain, type of surgery, lack of premedication, preoperative anxiety, surgery, awakening in a strange environment, and use of inhalation anesthesia, are recognized to affect EA (1, 4, 7). It has also been recommended that the quick and differential reduction of residual inhalation anesthetics might cause EA in operational patients, however others have shown that quick emergence is not a reason for EA after ending of anesthesia in the pediatric patient (8).
While EA is self-limited and may not lead to any lasting damage, it can result in self-harm and can be a reason for worrying of care providers and parents (9). Various techniques have already been recommended to reduce the occurrence and severity of EA, such as using sedative agents before induction, alteration of the maintenance propofol or injection of sedatives near the end of anesthesia (9, 10). Among these techniques, utilization of sedatives near the end of anesthesia is widely considered as the most convenient and also the most time-appropriate technique in clinical conditions, because it does not depend on the nature of the anesthetic components utilized throughout induction and also maintenance or the duration of anesthesia (11, 12). However, as these studies were performed independently and under different conditions by different evaluation tools, there is no proper comparison between midazolam and propofol available (13). The difference in mechanisms could influence recovery and incidence of complications. Several studies on the effect of Acetaminophen, Ketorolac, and Fentanyl on prevention of EA after sevoflurane anesthesia are done (6, 9, 14, 15). However, so far, no study is conducted on the effects of analgesic compounds on EA induced by Isoflurane.
2. Objectives
The present study, for the first time, compares the effects of midazolam and propofol on prevention of EA after maintaining anesthesia using Isoflurane, in children undergoing tonsillectomy.
3. Methods
3.1. Study Design
This is a clinical randomized double-blinded study, on children undergoing tonsillectomy, hospitalized at surgical unit of a university hospital. The parents consented in writing to participate in the study.
Sample size was calculated using the study by Aouad et al. (12) in which the mean and standard deviation for the pediatric anesthesia emergence delirium (PAED) in propofol group were reported as 8.6 and 3.9, and in normal saline group, 11.5 and 4.5 respectively. The significance level was assumed as below 0.05 level and the power of the study was taken as 0.8. Using the Power and Sample size program, sample size was calculated as 30 per each group. As no study in which there was a significant difference between midazolam and Propofolm was known, the sample size could not be calculated this way and therefore it was calculated using propofol and control groups, and the same number was used for the midazolam group.
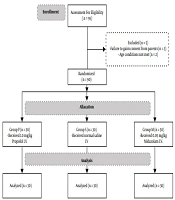
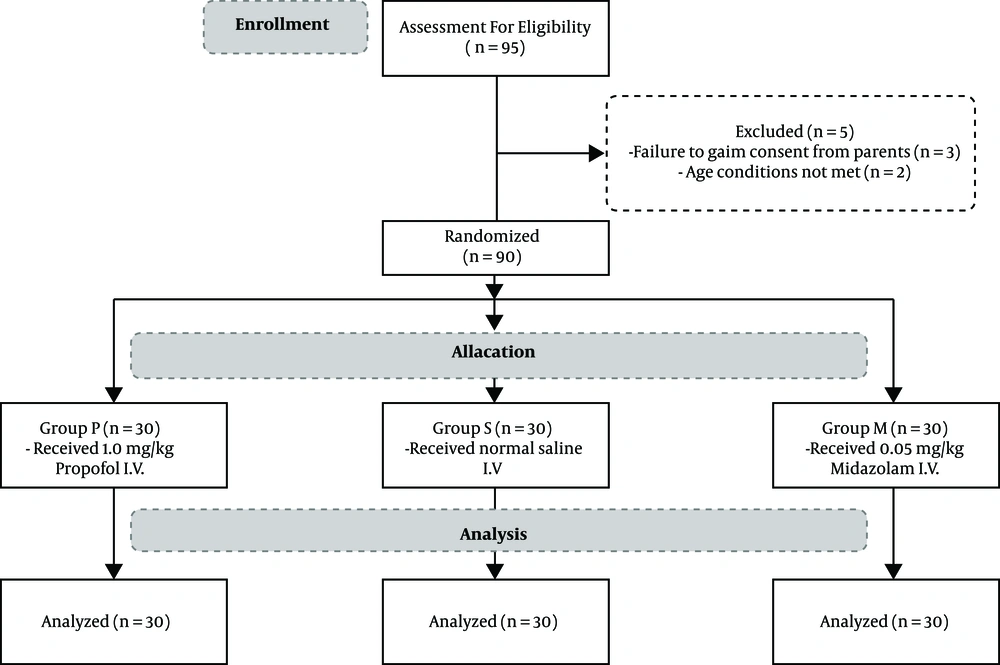
In this study, a total of ninety-five patients were assessed for eligibility, and ninety children aged 5 to 15, who met the inclusion criteria and were undergoing tonsillectomy, were randomly divided into three groups according to permutated block randomization, including P group (1.0 mg/kg propofol I.V.), M group (0.05 mg/kg midazolam I.V.), and S group (normal saline I.V.) after obtaining consent from their parents. In addition, the patients and the anesthesiology resident in charge of filling the PAED forms were not made aware of the medication given to each patient.
Inclusion criteria: Children 5 - 15 years of age, planned for tonsillectomy, ASA class I or II, Parents have given consent.
Exclusion criteria: Children with developmental delay, psychological, neurological or behavioral disorders, massive bleeding intra operative that leads to hemodynamic instability or transfusion. Figure 1 shows a flow diagram of this study.
After initial preparation and monitoring and initial serum therapy, following drugs were used for induction in all patients: Atropine 0.02 mg/kg, Fentanyl 2 µg/kg, sodium thiopental (STP) 6 mg/kg, Atracurium: 0.5 mg/kg.
Isoflurane was used in maintenance of anesthesia, as it is more common in use in operating rooms throughout Iran and cheaper compared to other volatile anesthetics, such as sevoflurane. On the other side, other agents, such as Halothane are of restricted access because of known side effects and are not common in use.
The drug was injected immediately after discontinuation of Isoflurane, and we measured the agitation rate at 5, 10, 20, and 30 minutes according to the PAED criteria. The degree of agitation upon awakening was evaluated and recorded at 5, 10, 20 and 30 minutes after emergence, the biggest values were then used for analysis. The incidence and severity of EA was calculated using PAED (16) scale (Table 1).
| Behaviors | None | A little | Quite a Bit | Very Much | Extremely |
|---|---|---|---|---|---|
| Eye contact with the caregiver | 4 | 3 | 2 | 1 | 0 |
| Actions are purposeful | 4 | 3 | 2 | 1 | 0 |
| Aware of his/her surroundings | 4 | 3 | 2 | 1 | 0 |
| Restlessness | 0 | 1 | 2 | 3 | 4 |
| Inconsolable | 0 | 1 | 2 | 3 | 4 |
3.2. Data Analysis
In quantitative variables, average and standard deviations were used for data description, and in qualitative variables, frequency and percentage were used. For data analysis chi-square test was used and t-test, ANOVA test and regression methods such as logistic regression were used if needed. All analyses were performed using SPSS software version 22.
4. Results
4.1. Demographic Characteristics
In the present study, all patients who were initially assessed successfully completed the study. No significant difference in gender, age, and weight among the three groups was found (Table 2).
| Parameter | Group P | Group M | Group S |
|---|---|---|---|
| Number of Patients | 30 | 30 | 30 |
| Age | 7.60 ± 2.36 | 8.73 ± 2.64 | 6.86 ± 2.69 |
| Gender, M/F | 20 (66.7)/10 (33.3) | 16 (55.3)/14 (46.7) | 22 (73.3)/8 (26.7) |
| Weight | 28.70 ± 14.64 | 36.07 ± 16.71 | 28.07 ± 31.46 |
4.2. Incidence of EA
EA was seen 60% (18/30) in group propofol (group P), 20% (6/30) in group midazolam (group M) and 97.6% (29/30) in group S, 5 minutes after being extubated. At 5 minutes, the incidence of EA in both M and P groups was significantly lower than that of group saline (group S) (P < 0.001 and P = 0.006 respectively), results also showed that EA incidence in group M was significantly lower than that in group P (P = 0.002). At other times the incidence of EA in groups M or P was significantly lower than that in group S but there was no significant difference between M and P groups. On the other hand, in all groups, incidence of EA has been higher at 5 minutes after extubation than other times. But the prevalence rate with the increase in its duration significantly decreased in groups P and S, while this difference was not seen in group M (Table 3).
Abbreviations: M, midazolam; min, minutes; P, propofol; S, saline.
aValues are expressed as No. (%).
bShowed a significant difference with group S.
cShowed a significant difference between M and P group.
dShowed a significant difference within a group at different times compared to 5 minutes after drug injection.
4.3. PAED Score
The mean values of PAED score at 5 minutes after drug injection in group P (10.30 ± 2.84) and group M (8.43 ± 3.65) were significantly lower than the values of group S (16.3 ± 2.61) (P < 0.001), but the difference between P and M groups did not reach statistical significance (P = 0.081). This means that the severity of agitation in midazolam group was significantly lower than that in S group. On the other hand, in all groups, PAED score was significantly higher at 5 minutes after extubation, the time in which the severity of agitation had somewhat reduced. Also, in different groups, the PAED score at different times showed a significant difference (P < 0.05), (Table 4).
Abbreviations: M, midazolam; min, minutes; P, propofol; S, saline.
aShowed a significant different with group S.
4.4. PACU and Other Parameters
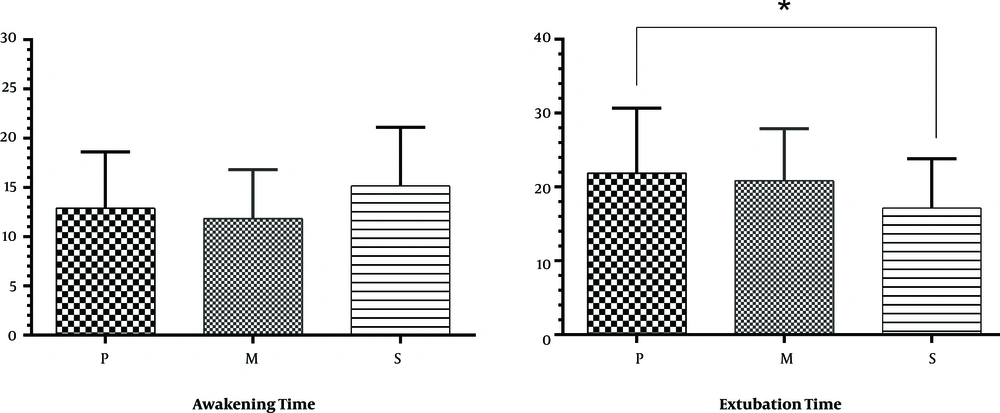
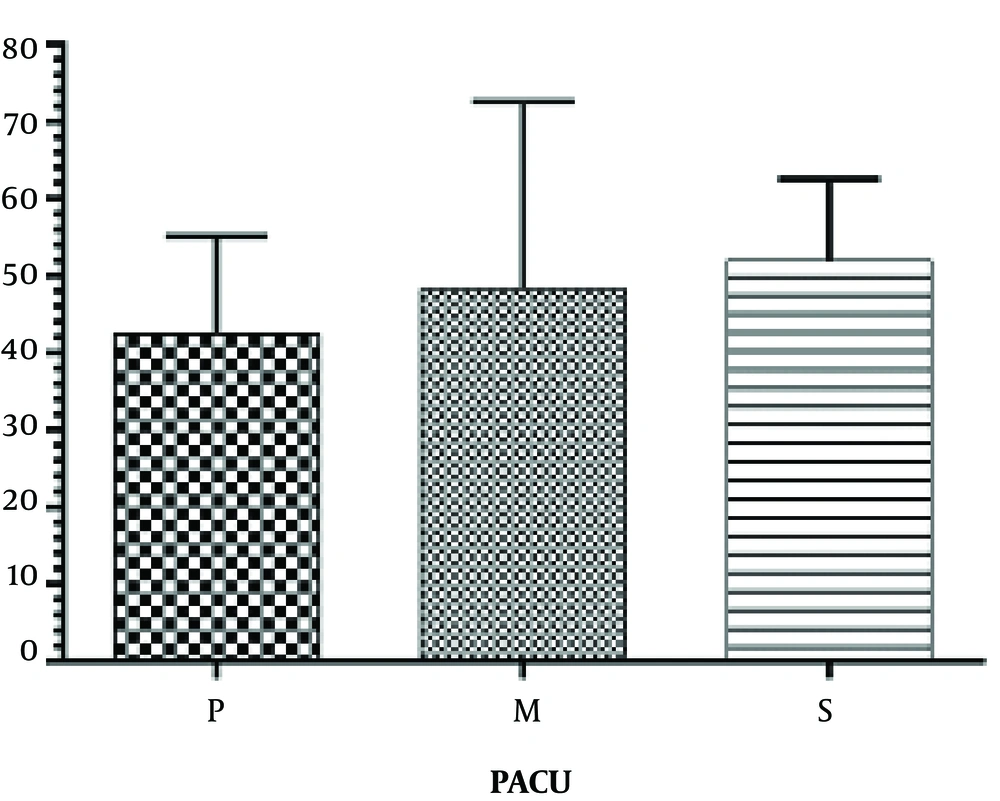
The extubation time (the time between the injection of drug and extubation) was prolonged in group P (21.9 ± 8.77) compared to group S (14.68 ± 6.65) (P < 0.001). No significant difference was found in the extubation time between group M (20.83 ± 7.08) with P and S groups. Also there were no significant differences for awakening time (the time between extubation and patient’s transfer to post-anesthesia care unit (PACU)) between all groups (Figure 2). No statistical difference was observed between groups P (42.50 ± 12.58) and M (48.33 ± 24.26), groups P and S (52.00 ± 10.64) and between groups M and S in PACU stay duration (P < 0.05) (Figure 3). Results showed no significant differences between all groups for apnea and laryngospasm (P > 0.05).
5. Discussion
In the present study, the effects of propofol and midazolam on EA were assessed in a group of patients who are at high risk for EA regarding to their age and the use of inhaled anesthetics and type of surgery. An EA incidence of 23.3% was observed in the control group. This rate compares to previous studies, which reported incidences between 16 and 34 percent (17, 18). Prophylactic use of i.v. propofol or midazolam reduced the incidence of agitation significantly to 3.3%, without adverse postoperative effects.
Numerous components of the anesthesia process have been inspected with the purpose of decreasing the incidence of EA with variable results (4, 6, 14-16). Propofol had been revealed to have a significant impact on prevention of EA. A study by Uezono et al. (9) suggested a reduction in EA risk by using a continuous propofol infusion while maintaining the anesthesia by using sevoflurane, and a 1 mg/kg dose of propofol administered at the end of operation, after sevoflurane has been discontinued, to decrease the incidence of agitation. Therefore, it is possible that the decreased incidence of EA accounted for the residual effect of propofol (19).
The impact of midazolam on EA remains debatable. Koner et al. found that midazolam premedication had no effect on EA following halothane or sevoflurane anesthesia (10). In another study, midazolam premedication decreased the incidence of EA (6). Chen et al. found that 0.05 mg.kg-1 midazolam combined with 0.5 μg.kg-1 of fentanyl at the end of surgery was effective in reducing the incidence and severity of EA. This result is expected because midazolam has a short duration of action that may not outlast the duration of the surgery (20). Kim et al. also compared propofol and midazolam in strabismus correction surgery. They found that midazolam and propofol reduced the rate of EA by about 40%, resulting in a prophylactic incidence of 40%, still higher than that of Chen (19, 21).
Several researchers have disclosed that midazolam decreases EA by acting on its target effect site GABAA, and also its antagonist, flumazenil reverses this. Nevertheless, the exact mechanism remains unknown, in fact it is not recognized whether sevoflurane and midazolam interact at the GABAA receptor level (6, 20).
The PAED scale, used for the assessment of EA, is a known tool for evaluating EA, the reliability and validity of which has been previously proved (22-25). While some recent studies (26) propose the use of age-adjusted PAED scores, no such score was used in this study. Using the PAED scale, midazolam and propofol showed comparable effectiveness in preventing EA.
This study faced several limitations. First, we evaluated only children undergoing one specific type of surgery, namely tonsillectomy. The occurrence of EA is different based on the type of operation, assuming that the efficiency of midazolam and propofol in this study may differ in other operations. Secondly, midazolam and propofol doses utilized in this study are only arbitrary. This, in part is because of the fact that there is no dose of midazolam and propofol similar in potency for elimination of EA induced by Isoflurane. Therefore, a propofol dose of 1 mg/kg was used, similar to the study by Aouad et al. (12) who used this dose for prevention of EA in anesthesia induced by sevoflurane. It had already been demonstrated that in the pediatric patient, sedation with i.v. midazolam (0.05 to 0.1 mg/kg) is safe and effective for invasive or lengthy procedures (19). Therefore, a minimum sedation dose of 0.05 mg/kg for midazolam was used in this study.
5.1. Conclusions
In conclusion, administration of either midazolam or propofol near the end of surgical operation may be effective in reducing the severity and incidence of EA in the pediatric patient undergoing tonsillectomy after anesthesia by Isoflurane and neither is significantly more effective than the other.