1. Context
Chronic fatigue syndrome (CFS) or systemic exertion intolerance disease (SEID) (1) has a prevalence of 0.2% to 2.6% and is characterized by recurrent or persistent fatigue for at least 6 months without any medical explanation (2). This syndrome occurs more frequently in females than males (3). The etiology of this disease is unknown, yet symptoms include a variety of somatic symptoms, impairment of neurocognitive functions, and quality of sleep. The origin of the disease is suggested to be a viral infection in some studies (4) and exacerbation of CFS seems to be caused by viral and non-viral agents (5). The diagnostic criteria of CFS, as defined by the Institute of Medicine, include a decline in personal or occupational activities for more than 6 months, post exertional malaise, and unrefreshing sleep with at least 2 of the following signs: cognitive impairment or orthostatic imbalance (6).
Several viruses are proposed to play a role in the etiology of CFS, such as retroviruses, human herpes virus type 6 (HHV-6), Enteroviruses, Coxsackie B disease virus, Adenovirus, Ross river virus, and Borna disease virus (BDV) (7, 8).
BDV is an enveloped, non-segmented, negative-strand RNA virus from Bornaviridae family and order Mononegavirales (9). The BDV can lead to neuronal chronic slow progressive infections and occasionally involve glial cells in vivo and could lead to the involvement of several classes of cells in vitro (10). Numerous studies have been conducted on the effect of BDV on several psychiatric and neurological disorders. Results of a meta-analysis conducted by Isabel Arias et al. in 2012 showed an association between BDV and schizophrenia (P < 0.01) (11). Miranda et al. compared psychiatric patients with a control group and reported a higher rate of BDV RNA in the patients (12) and Puerto confirmed the possible role of BDV (through Western Blot [WB] diagnostic method) in neuropsychiatric diseases (13). Another study also reported the association between BDV and encephalitis (through diagnostic methods of WB and RT-PCR [reverse transcription polymerase chain reaction]), yet there was no association with other neurological disorders and controls (14). However, one meta-analysis study did not confirm the association between human BDV infections and psychiatric diseases (15). In addition, Indirect Immunofluorescence Antibody (IFA) and rRT-PCR tests rejected the possible role of BDV in patients with psychiatric diseases (16).
Regarding the association between BDV and CFS, controversial results have been reported by previous studies. In a study by Bode et al. the role of BDV in the etiology of CFS was no significant (17). Evengardand et al. did not support the role of BDV in the pathogenesis of CFS (18). In a Japanese study by Nakaya et al. the prevalence of BDV p24RNA and antibody in Peripheral Blood Mononuclear Cells (PBMCs) and serum of patients with CFS was 32%, supporting the association between BDV and CFS (19). Some studies suggested a strong association in some cases of CFS (20, 21).
2. Objectives
Because of the controversy on this issue, a systematic review and meta-analysis is essential to review all the relevant documents and come to a final conclusion (22, 23). Considering the fact that CFS has a wide range of symptoms that are common among different disorders, CFS has several differential diagnoses, and so it is important to diagnose CFS. Moreover, the underlying mechanism or etiology of CFS has not been well established. Thus, identifying the possible etiologic agents could help in the diagnosis of this disabling disease. One of the probable etiologies is BDV, yet, the role of BDV in patients with CFS has not been proven. Therefore, we conducted a systematic review and meta-analysis to assess the effect of this infectious agent on the etiology of CFS.
2.1. Data Source
The present systematic review and meta-analysis was conducted using preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (24). To avoid bias, all procedures of the current study were performed by two researchers independently.
3. Evidence Acquisition
3.1. Information Sources and Search Strategy
A comprehensive search on the following databases was performed until December 2016: PubMed, Scopus, Embase, PsycINFO, Science Direct, Cochrane Library, web of science and Google scholar search engine to identify studies addressing the association between CFS and BDV. The online search consisted of the following MeSH keywords: Borna disease virus, chronic fatigue syndrome, neurological disorders, and neuropsychiatric disorders. In addition, a reference list of relevant articles was evaluated to access additional articles related to the study objectives.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were epidemiologic studies addressing the association between BDV and CFS with at least an English abstract. Exclusion criteria were: 1. Irrelevant to topic; 2. Not chronic fatigue syndrome patients in case group; 3. Not assessing Borna disease virus; 4. Conference presentations, letters to editors and review article.
3.3. Study Selection
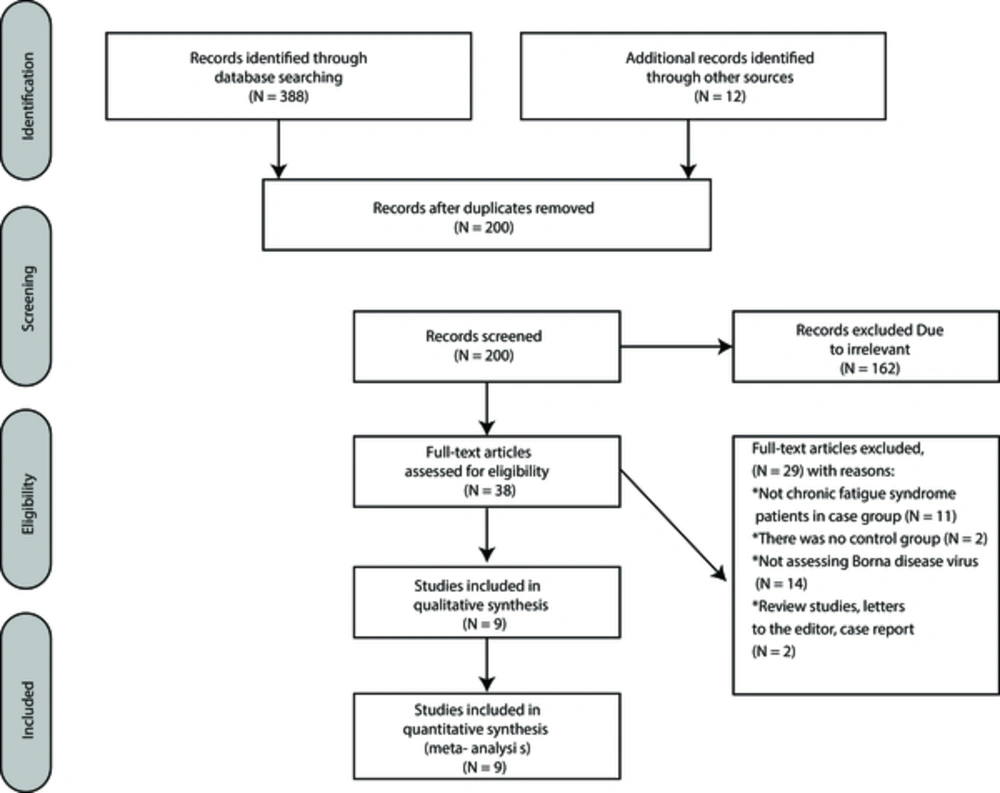
The initial search resulted in 400 studies, and 200 duplicate articles were excluded from our research by EndNote software. After screening the title and abstract of all articles, 38 studies were selected for full-text assessment. Finally, 9 studies were included in quantitative synthesis (Figure 1).
3.4. Quality Assessment
The selected articles were reviewed by two researchers using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (25). The checklist contains 22 items. Each item was given a score between 0 and 2 and the data that gained a score above 16, entered the analysis.
3.5. Data Extraction
Two researchers independently reviewed all studies that were entered in the analysis process. The following characteristics were extracted and recorded for each paper: author(s) name, country of study, year and design of study, BDV measure (PBMCs, Plasma, and Serum), name of journal, characteristics for patients and controls (e.g. gender and mean age and SD, and size), diagnostic methods, diagnostic criteria, odds ratio, number of positive BDVs in cases and controls. Disagreement between researchers was resolved by a third researcher.
3.6. Meta-Analysis
We pooled odds ratios (ORs) and 95% confidence intervals (CI). Heterogeneity between studies was measured by Cochran's Q test and I2 index using forest plots of OR. P values < 0.05 for Cochran's Q test or I2 values >50% were considered as heterogeneity (26). Cochran's Q test showed P=0.03 and I2 index was 62%. Since the heterogeneity was apparent, we used the random effects model to combine studies. Data was analyzed using Review Manger software version 5.3.5. P < 0.05 was considered statistically significant. To explore potential sources of heterogeneity, we conducted subgroup analysis for different methods and assays. Publication bias was assessed by Egger’s and Begg’s tests using comparative meta-analysis (CAM) software ver. 2.
4. Results
4.1. Characteristics of the Study Populations
Nine studies with a total sample size of 610 cases and 2176 controls were obtained for the meta-analysis (Table 1).
| Authors Name | Year | Place | Case | Control | Assays | Technique/Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive BDV, N | Negative BDV, N | Male/Female | Mean age (SD) | Positive BDV, N | Negative BDV, N | Male/Female | Mean age (SD) | |||||
| Yamaguchi et al. (31) | 1999 | Japan | 0 | 75 | 45/30 | 32 (9.8) | 10 | 907 | 632/285 | 35.1 (13.1) | anti p24 and p40 Ab | ECLIA/Serum |
| Kitani et al. (27) | 1996 | Japan | 30 | 59 | - | 32.6 | 3 | 97 | - | 37.9 | anti p24 Ab | ELISA/Serum |
| Nakaya et al. (20) | 1999 | Japan | 7 | 0 | - | - | 1 | 2 | - | - | anti p24, p40 and gp18 Ab | Immunobloting/Plasma |
| Evengard et al. (18) | 1999 | Sweden | 27 | 142 | - | - | 2 | 60 | - | - | anti BDV p40,p24,gp18Ab | ELISA/Serum |
| Li Yj et al. (28) | 2003 | China | 7 | 54 | - | - | 0 | 73 | - | - | anti p24Ab | WB/Plasma |
| Li Yj et al. (29) | 2005 | China | 17 | 65 | - | - | 0 | 73 | - | - | anti p24Ab | WB/Plasma |
| Kitani et al. (27) | 1996 | Japan | 7 | 50 | - | - | 8 | 164 | - | - | P24RNA | Nested RT_PCR/PBMCs |
| Nakaya et al. (19) | 1996 | Japan | 6 | 1 | - | - | 0 | 3 | - | - | P24RNA | Nested RT_PCR/PBMCs |
| Zhang et al. (21) | 2013 | China | 8 | 55 | 38/25 | 37.4 (10.8) | 6 | 767 | 426/347 | 40.5 (8.5) | p24 or p40RNA | Nested RT_PCR/PBMCs |
4.2. Overall Association Between BDV and CFS
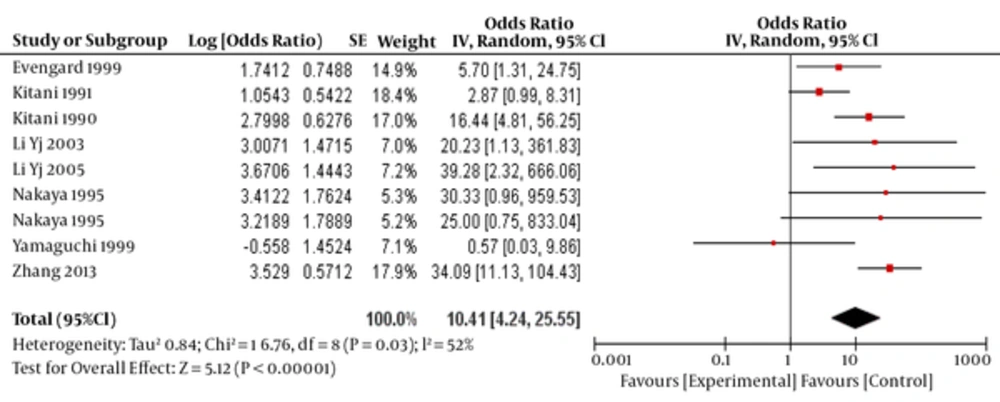
The total OR was estimated to be 10.41 (95% CI:4.24-25.55), which was statically significant (P < 0.0001)(Figure 2).
4.3. Subgroup Analysis Based on Different Methods
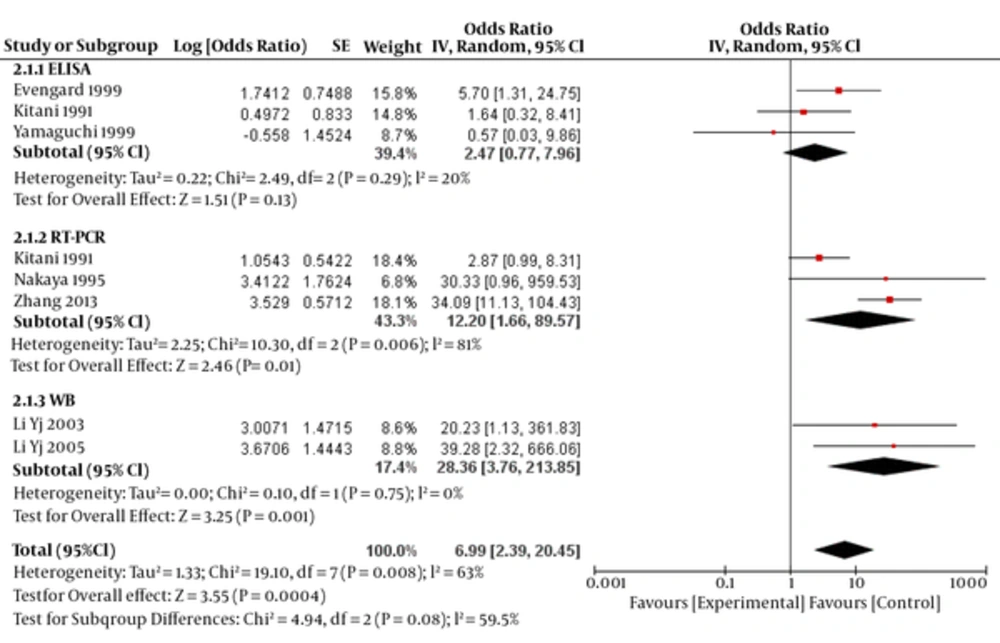
OR for ELISA, RT-PCR and WB methods was estimated to be 2.47 (95% CI: 0.77- 7.96, P=0.13), 12.20 (95% CI: 1.66- 89.57, P=0.03) and 28.36 (95% CI: 3.76-213.85, P=0.001), respectively (test for subgroup differences: P=0.08; I2=59.5 (Figure 3).
4.4. Subgroup Analysis Based on Different Assays
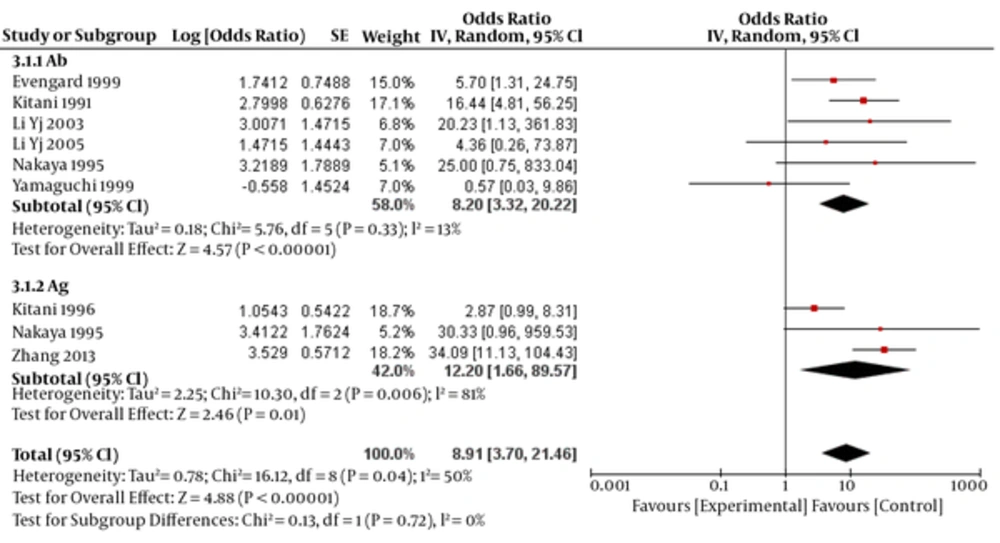
OR for assays of antigens and antibodies was estimated to be 12.20 (95% CI: 1.66- 89.57, P < 0.0001) and 8.20 (95% CI: 3.32- 20.22, P=0.01), respectively (test for subgroup differences: P=0.72; I2=0 (Figure 4).
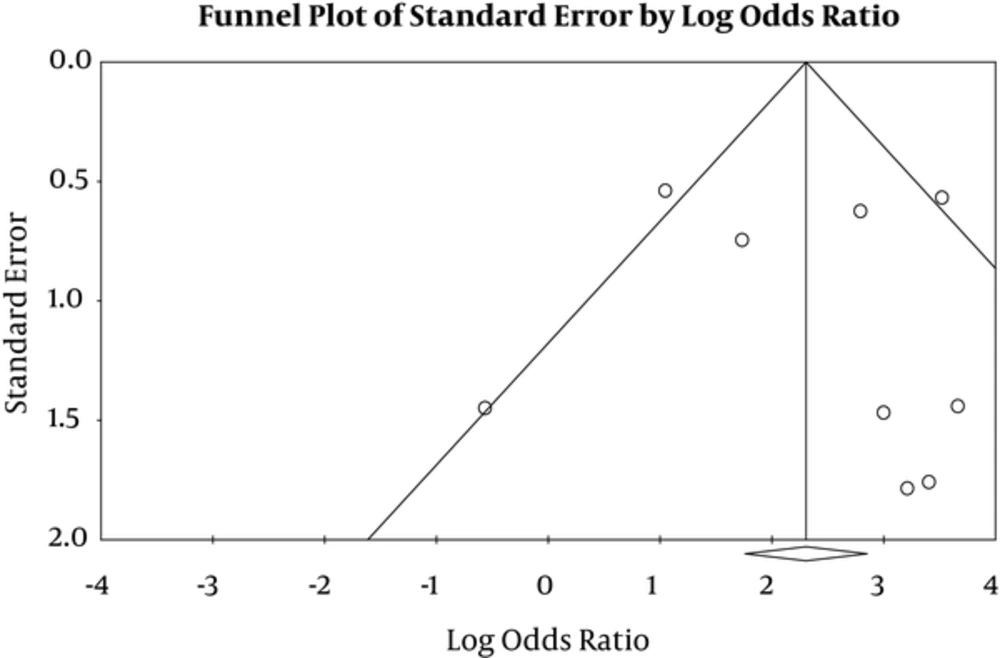
4.5. Publication bias
P-value for publication bias in Egger’s and Begg’s tests was estimated to be 0.41 and 0.50, respectively, which was not statically significant (Figure 5).
5. Discussion
In the present study, after searching databases and the process of study selection and quality assessment, 9 studies were finally analyzed, which resulted in a total OR of 10.41 (95% CI: 4.24 to 25.55, P < 0.0001). The OR for most of these 9 studies was significant, indicating BDV as a strong etiologic agent that influences CFS. However, the heterogeneity of studies was moderate (52%) (P = 0.03), and therefore, random effects model was used in the meta-analysis (to investigate heterogeneity according to I2 index, there are three classifications: less than 25% [low heterogeneity], 25-75% [moderate heterogeneity] and more than 75% [high heterogeneity]); the analysis was performed according to diagnostic methods and assays, was no significant (P = 0.08, and 0.72, respectively).
Among these 9 studies, 2 studies did not find an association between BDV and CFS. Evengard et al. reported that in Swedish patients, there was nonspecific immunoreactivity in ELISA to BDV in CFS sera (due to infectivity with other microbes or viral agents, as well as being bound to b-galactosidase), which was not confirmed by WB; in addition to RT-PCR detection of P-gene transcripts in PBMC (18). However, the geographic differences and other factors could be the reason for this discrepancy. The studies that found no association declared that failure to find an association does not mean lack of any kind of association and suggested further studies.
However, studies had a moderate discrepancy and accurate meta-analysis should be performed on the gene-bank of the patients, rather than the studies published to minimize the confounders and differences of studies (30). Moreover, it has been posited that BDV might be positive only in the acute phase of CFS disease (32).
The present study had some limitations including issues with access to full text of all articles, using different methods (WB, RT-PCR, ELISA), samples (Serum, Plasma, PBMCs) and assays (anti bodies, antigens) for detecting BDV in patients with CFS in different studies. However, the strength of our study was using subgroup analysis of studies according to different methods and assays.
This study supports the association between BDV and CFS and shows the role of viral agents in the etiology of. Therefore, viral agents may play a role in the etiology of neuropsychiatric disorders. By understanding the underlying factors of a psychiatric disease, we can take a crucial step forward to its treatment on a medical basis and decrease the prevalence of uncured cases with neuropsychiatric disorders, including treatment of infectious disease to help health programs.
Further studies are required to find the exact association and to find other etiologic agents. In addition, a high-quality technique needs to be used for detection of BDV.