1. Background
Renal cell carcinoma (RCC) is the third most common cancer of the genitourinary system and represents approximately 3% to 5% of all adult malignancies (1). Currently, RCCs are frequently diagnosed at clinical T1a because of the widespread use of abdominal imaging (2). Furthermore, with the advancements in laparoscopic and robot-assisted techniques, minimally invasive nephron-sparing surgery has been increasingly adopted for management of small renal masses. Compared to total nephrectomy, in case of nephron-sparing surgery, it is important to identify preoperative anatomic features of the kidney, renal vessels, and the tumor because of complexity of the technique. Therefore, the role of cross-sectional imaging in the treatment of small renal masses has diversified from detection and staging of tumors to prediction of operative complexity before surgery. Traditionally, operative complexity evaluated with the major emphasis on tumor-related anatomic factors, including tumor size, location, centrality, tumor deepness into the parenchyma, and nearness of the tumor to the sinus, which are described using the RENAL (radius, exophytic/endophytic properties, nearness of tumor to the collecting system or sinus in millimeters, and anterior/posterior location relative to polar lines) nephrometry score (3). Since the first description of the RENAL nephrometry scoring system in 2009, more than 10 nephrometry scoring systems have been published (4). However, studies have shown that even during operation of masses under similar conditions of tumors, various difficulties can be experienced during dissection of fat tissues (5). Although the body mass index (BMI) has been used to evaluate visceral fat, it has been reported to have little association with complications in partial nephrectomy (PN) (6, 7). Several studies suggested that thick, adherent “sticky fat” could be difficult to dissect during surgery, and thus, the presence of adherent perinephric fat (APF) should be included in the prediction of operative complexity. The Mayo adhesive probability (MAP) score was developed based on the results of a study that showed APF could be predicted using preoperative CT images (8). In this paper, the method of measurement of posterior perinephric fat thickness is simply referred to as “posterior fat was measured as a direct line posteriorly from the renal capsule to the posterior abdominal wall”.
Various studies have reported the clinical significance of APF. During PN, APF has been identified to be associated with various perioperative outcomes, including increased bleeding and a higher risk of conversion operation (5) or longer operative time (9), and with decreased progression-free survival in localized RCC (10). However, from the perspective of a radiologist, the method used for measuring perinephric fat thickness was considered as excessively simple, which may lead to low reproducibility. To increase the availability of nephron-sparing surgery, which has the advantage of preserving the patient’s renal function, it is necessary to develop an objective scoring system that predicts operative complexity before surgery.
2. Objectives
The purpose of this study was to investigate reproducible measurement methods of perinephric fat with comparison of two tailored methods for the measurement of PPFT on preoperative CT and examine the association between the methods and operative complexity in PN.
3. Patients and Methods
3.1. Patient Selection
This cross-sectional study received approval from the institutional review board, and the requirement for informed consent was waived. From September 2012 through August 2016, 75 patients (58 men and 17 women) underwent PN performed by a single experienced surgeon (BLINDED) at BLINDED Hospital. Among 75 patients, three patients were excluded due to the absence of pathologic result (n = 1) and lack of record for ischemia time (n = 2). Finally, 72 patients (56 men and 16 women; age range, 32 - 89 years) were enrolled in the present study. Of the 72 patients, 44 underwent robotic-assisted surgery and 28 underwent open surgery. Data on operative time and ischemia time, pathologic results, and BMI were obtained from medical chart reviews. Operative time and ischemia time were used as reference standards to reflect operative complexity.
3.2. CT Technique
Preoperative CT scans acquired within 1 month of surgery were available for all included patients. All CT examinations were performed on one of three scanners: a 64-channel CT scanner (Brilliance 64; Philips Medical Systems, Cleveland, OH [detector collimation, 64 × 0.625 mm; reconstruction, 3- or 5-mm slice thickness; 120 kV; and 120 - 280 mAs]) and two 128-channel CT scanners (SOMATOM Definition AS1; Siemens, Erlangen, Germany [detector collimation, 6430.6 mm using z-flying focal-spot technology; reconstruction, 3- or 5-mm slice thickness; 100 kV; and 100 - 350 mAs] and SOMATOM Definition Flash; Siemens [detector collimation, 12830.6 mm; reconstruction, 3- or 5-mm slice thickness; and 100-kVp tube voltage using online dose modulation] with CARE Dose4D; Siemens). CT images were acquired after administration of 2 mL/kg body weight of non-ionic iodinated contrast material (iobitridol [Xenetix 300; Guerbet, Villepinte, France] or iopamidol [Pamiray 300; Dongkook Pharmaceutical, Seoul, South Korea]) by using a power injector at a rate of 2.5 - 3.0 mL/s.
3.3. Image Analysis
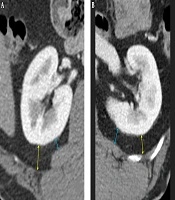
In the MAP score (8), measurement of posterior perinephric fat thickness (PPFT) was described as “drawing a direct line posteriorly from the renal capsule to the posterior abdominal wall at the level of the renal vein”. Therefore, in the first review session, the two blinded reviewers, with 7 and 23 years of experience of abdominal CT interpretation, measured PPFT according to the PPFTMayo method without training. After completing the first review session, one study coordinator (BLINDED with 11 years of experience in abdominal CT interpretation) provided training to the two reviewers on the tailored methods for evaluating PPFT to overcome the ambiguity of the MAP scoring system. Thereafter, in the second review session, which was performed more than 2 weeks after the first session, PPFT was measured using the two different methods. On the basis of the findings of a recent study showing maximal association between the MAP score and posterolateral perinephric fat thickness (11), PPFTcostal was defined as the longest distance measured by drawing a line towards the posterolateral direction from the capsule to the transverse abdominis muscle, intercostal muscle, or their tendons, perpendicular to the capsule. PPFTlumborum was defined as the shortest distance that was measured by drawing a line from the capsule to the quadratus lumborum muscle, perpendicular to the capsule (Figure 1). PPFT was measured at the level where the ipsilateral renal vein was largest. Perinephric fat stranding was defined as linear or curvilinear soft-tissue attenuation without a vascular connection and with distribution in the perinephric space. Gerota’s fascia was used as a generic term to describe both anterior and posterior pararenal fasciae. On CT images, the following three-point scale was defined to score severity: 0 = no stranding; 1 = focal stranding; and 2 = diffuse or multifocal stranding or presence of Gerota’s fascia thickening (Figure 2). The RENAL nephrometry scoring system, which refers to the five elements that constitute the operative complexity of the tumor, was used for stratifying the complexity of renal masses as previously used and described in the literature (12). Two reviewers independently assessed PPFT, the perinephric stranding score, and the RENAL nephrometry score. Reviewers magnified the selected image and evaluated using image archiving and communication system (INFINITT PACS, version 3.0; INFINITT Healthcare, Seoul, Korea).
3.4. Statistical Analysis
We performed statistical analyses by using IBM SPSS Statistics for Windows/Macintosh, version 20.0 (IBM Corp., Armonk, NY). Interobserver agreement between the two reviewers for PPFT, the perinephric fat stranding score, and the RENAL nephrometry score was analyzed using kappa statistics or the intraclass correlation coefficient (ICC). The strength of agreement was defined based on the kappa or ICC value as poor (< 0.20), fair (0.21 - 0.40), moderate (0.41 - 0.60), substantial (0.61 - 0.80), or excellent (0.81 - 1.00). The ICCs were compared between the groups using Fisher’s z-test (13). The measured data, baseline characteristics, and ischemia time and operative time were compared between the groups using the χ2 test, Fisher’s exact test, and Mann-Whitney U-test. A multiple linear regression analysis with stepwise variable selection method was used to evaluate the effect of age, sex, body mass index, pathology, laterality, PPFT, perinephric stranding and nephrometry score on operative time and ischemia time in open and robotic-assisted PN. A P value < 0.05 was considered statistically significant.
4. Results
Demographic data of the patients who underwent open or robotic-assisted PN are listed in Table 1. Ischemia time and operative time were significantly shorter in open PN (mean, 19.1 ± 6.9 min and 3.1 ± 0.6 h, respectively) Thani n robotic assisted PN (mean, 25.3 ± 7.4 min and 3.5 ± 0.7 h, respectively) (P < 0.001 and 0.034, respectively). Patients who underwent open PN were significantly older than those who underwent robotic-assisted PN. Measured data including PPFT, perinephric fat stranding score, RENAL nephrometry score, sex, BMI, pathologic results, and tumor laterality showed no significant differences between the two groups.
| Open partial nephrectomy | Robotic-assisted partial nephrectomy | P value | |
|---|---|---|---|
| PPFTcostal, mm | |||
| Reviewer 1 | 13.57 ± 7.74 | 14.28 ± 8.91 | 0.777 |
| Reviewer 2 | 13.77 ± 7.55 | 14.46 ± 8.64 | 0.725 |
| PPFTlumborum, mm | |||
| Reviewer 1 | 6.51± 6.99 | 7.91 ± 7.08 | 0.301 |
| Reviewer 2 | 7.07± 6.77 | 8.14 ± 7.16 | 0.560 |
| Perinephric stranding | |||
| Reviewer 1 | 14:12:2 | 21:21:2 | 0.854 |
| Reviewer 2 | 12:14:2 | 19:23:2 | 0.893 |
| RENAL nephrometry score | |||
| Reviewer 1 | 6.36 ± 1.254 | 6.48 ± 1.455 | 0.943 |
| Reviewer 2 | 6.39 ± 1.197 | 6.55 ± 1.547 | 0.845 |
| Ischemia time, min | 19.0714 ± 6.89567 | 25.3089 ± 7.42679 | < 0.001 |
| Operative time, h | 3.1450 ± 0.63051 | 3.4755 ± .69214 | 0.034 |
| Age, y | 61.00 ± 12.881 | 52.57 ± 11.300 | 0.008 |
| Sex (male: female) | 20:8 | 36:8 | 0.386 |
| BMI | 25.8010 ± 3.40495 | 25.2816 ± 3.05726 | 0.368 |
| Pathology (malignant: benign) | 26:2 | 39:5 | 0.698 |
| Laterality (right: left) | 16:12 | 24:20 | 1.000 |
Abbreviations: BMI, body mass index; PPFT, posterior perinephric fat thickness; PPFTcostal, the longest distance measured by drawing a line towards the posterolateral direction from the capsule to the transverse abdominis muscle, intercostal muscle, or their tendons, perpendicular to the capsule; PPFTlumborum, the shortest distance that was measured by drawing a line from the capsule to the quadratus lumborum muscle, perpendicular to the capsule; RENAL, radius, exophytic/endophytic properties, nearness of tumor to the collecting system or sinus in millimeters, and anterior/posterior location relative to polar lines.
aValues are expressed as mean ± SD.
Interobserver agreement between the two reviewers for PPFT was excellent (ICC, 0.854; 95% confidence interval [CI]: 0.767 - 0.910) when measured using PPFTMayo; excellent (ICC, 0.965; 95% [CI]: 0.944 - 0.978) when measured using PPFTcostal; and excellent (ICC, 0.980; 95% [CI]: 0.970 - 0.988) when measured using PPFTlumborum. The ICCs between the two reviewers when using PPFTcostal and PPFTlumborum showed no statistical differences (P = 0.173, Fisher’s z-test); however, the ICCs when using the two tailored methods, including PPFTcostal and PPFTlumborum, were significantly higher than that when using PPFTMayo (P < 0.001 and P < 0.001, respectively). Interobserver agreement between the two reviewers was also excellent (ICC, 0.921; 95% [CI]: 0.877 - 0.950) for the RENAL nephrometry score and substantial (kappa value, 0.698; 95% [CI]: 0.671 - 0.775) for the perinephric fat stranding score.
The results of the multiple linear regression are shown in Table 2. It was observed that the RENAL nephrometry score was a determinant of ischemia time (P < 0.001 or 0.001) and PPFT was a determinant of operative time (P = 0.009 ~ 0.023) in robotic-assisted PN for both the reviewers. In open PN, the RENAL nephrometry score and BMI were identified as the determinants of ischemia time (P = 0.002 ~ 0.014). However, analysis of operative time showed different results between the two reviewers; the RENAL nephrometry score was observed as a determinant in reviewer 1 (P = 0.033) and sex was observed as a determinant in reviewer 2 (P = 0.049).
| Type of partial nephrectomy | Method for measuring PPFT | Dependent variables | Reviewer 1 | Reviewer 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistically significant variables | B | SE | Standardized Regression coefficient | P-value | Statistically significant variables | B | SE | Standardized Regression coefficient | P-value | |||
| Robotic-assisted | PPFTcostal | Ischemia time | Nephrometry | 2.408 | 0.695 | 0.472 | 0.001 | Nephrometry | 2.429 | 0.639 | 0.506 | < 0.0001 |
| PPFTlumborum | Nephrometry | 2.408 | 0.695 | 0.472 | 0.001 | Nephrometry | 2.429 | 0.639 | 0.506 | < 0.0001 | ||
| PPFTcostal | Operative time | PPFT | 0.027 | 0.011 | 0.343 | 0.023 | PPFT | 0.031 | 0.011 | 0.39 | 0.009 | |
| PPFTlumborum | PPFT | 0.036 | 0.014 | 0.372 | 0.013 | PPFT | 0.037 | 0.014 | 0.383 | 0.01 | ||
| Open | PPFTcostal | Ischemia time | Nephrometry | 2.634 | 0.875 | 0.479 | 0.006 | Nephrometry | 3.075 | 0.872 | 0.534 | 0.002 |
| BMI | 0.854 | 0.322 | 0.422 | 0.014 | BMI | 0.827 | 0.306 | 0.408 | 0.012 | |||
| PPFTlumborum | Nephrometry | 2.634 | 0.875 | 0.479 | 0.006 | Nephrometry | 3.075 | 0.872 | 0.534 | 0.002 | ||
| BMI | 0.854 | 0.322 | 0.422 | 0.014 | BMI | 0.827 | 0.306 | 0.408 | 0.012 | |||
| PPFTcostal | Operative time | Nephrometry | 0.203 | 0.09 | 0.404 | 0.033 | Sex | -0.496 | 0.24 | -0.362 | 0.049 | |
| PPFTlumborum | Nephrometry | 0.203 | 0.09 | 0.404 | 0.033 | Sex | -0.496 | 0.24 | -0.362 | 0.049 | ||
Abbreviations: B, Un-standardized regression coefficient; BMI, body mass index; CI, confidence interval; PPFT, posterior perinephric fat thickness; PPFTcostal, the longest distance measured by drawing a line towards the posterolateral direction from the capsule to the transverse abdominis muscle, intercostal muscle, or their tendons, perpendicular to the capsule; PPFTlumborum, the shortest distance that was measured by drawing a line from the capsule to the quadratus lumborum muscle, perpendicular to the capsule; SE, standard error.
aCovariates included in a multiple linear regression model were age, sex, body mass index, pathology, laterality, perinephric stranding, posterior perinephric fat thickness and nephrometry score, and only the covariates which showed statistical significance are written in a table. The dependent variables in each model were the continuous variables, ischemia time and operative time.
5. Discussion
Traditional nephrometry scoring systems, such as the RENAL score and the preoperative aspects and dimensions used for an anatomical (PADUA) score, could be used to predict the complexity of PN by focusing on renal morphometry (3, 14). However, these nephrometry scoring systems do not take into account the patient-specific factors, which lead to complications in the technical aspects of PN. The BMI is the simplest estimate of obesity but does not specify a patient’s relative distribution of abdominal wall fat and fat surrounding the internal organs. Several previous studies have demonstrated that perinephric fat is a stronger determinant of operative complexity than is the BMI in patients undergoing robotic-assisted PN (15, 16). Because robotic-assisted PN is mostly performed via the transperitoneal approach, subcutaneous fat no longer acts as an obstacle after the robotic instrument enters the abdominal cavity, while the amount of perinephric fat is crucial in dissecting the renal hilum and exposing the tumors. In particular, because the increased thickness of posterior perinephric fat was shown to be associated with higher operative complexity (8, 15), PPFT was included in the MAP score to assess operative complexity by analyzing CT images. Based on the authors’ experience, however, the method of measuring PPFT proposed in the MAP scoring system appears as ambiguous and less reproducible because of the bumpy surface of the posterior boundary of perinephric fat, which is made up of the quadratus lumborum muscle and abdominal wall muscles including the transverse abdominis muscle, intercostal muscle, or their tendons. In addition, it is hypothesized that the reproducibility of the measurement may further be reduced because of various angles between the wall and the kidney. To overcome this problem, our study described a more detailed measurement method. As the quadratus lumborum muscle was relatively flat or convex and narrow at the back of the kidney, it was considered as a suitable and reproducible target to measure the shortest distance perpendicular to the renal capsule for measuring PPFTlumborum. On the contrary, since the inner margin of the abdominal wall, consisting of the transverse abdominis muscles, intercostal muscles, or their tendons, was concave and widely located at the posterior and posterolateral aspects of the kidney, it was considered as an accurate target to measure the longest distance for measuring PPFTcostal. Our study has shown that when PPFT was measured using these detailed methods, the strength of agreement between the two reviewers was significantly higher than that measured using the method presented in the MAP scoring system. For these two tailored methods, the strength of agreement between the two reviewers was excellent without any statistical difference between the ICCs for measuring PPFTlumborum and PPFTcostal. Consequently, both methods devised in this study were observed to be suitable for measuring PPFT.
Investigation of the determinants predicting operative complexity using PPFTlumborum and PPFTcostal in robotic-assisted PN revealed that PPFT was associated with operative time and RENAL nephrometry score was associated with ischemia time. These results are in agreement with the ones published in previous studies (9, 17, 18). On the contrary, in open PN, only the nephrometry score was identified as a relevant factor predicting ischemia time for both the reviewers and operative time for one of the reviewers. For another reviewer, neither PPFT nor the RENAL nephrometry score was related to the two parameters reflecting operative complexity. It is known from previous studies that the nephrometry score in open PN is related to various perioperative outcomes (19), but the effect of perinephric fat-related factors as per the different methods of PN has not been studied. The difference in the effect of PPFT on operative complexity in two different surgical methods can be explained by the difference in surgical approach. In robotic-assisted surgery, a small incision is made, and dissection is performed to expose the tumor and hilum using robotic instruments, which leads to technical difficulties in patients with large amounts of perinephric fat tissue. However, in open surgery, fat could be dissected in a relatively free manner from the surrounding tissues through wide exposure, which does not seem to affect operative time.
Perinephric fat stranding has been reported as the determinant for predicting operative complexity in the MAP score along with PPFT (8), and the underlying pathophysiology of perinephric “sticky fat” has been thought to be inflammation, desmoplasia, idiopathic fibrosis, or autoimmune response (20). However, from a radiological perspective, the cause of perinephric fat stranding varies from acute to chronic, with a wide spectrum of conditions including acute ureteral obstruction, pyelonephritis, bladder outlet obstruction, postoperative change, acute pancreatitis, and metastasis (21-24). Moreover, in a histopathologic comparison of patients with or without APF, Dariane et al. (17) demonstrated no significant difference in inflammatory infiltration or fibrosis in the perinephric tissue but only significantly larger adipocytes in patients with APF and concluded that the histology of adhesive perinephric fat was unclear. In the present study, the degree of perinephric fat stranding was not associated with operative or ischemia time, thereby revealing its irrelevance in predicting operative complexity. Therefore, it may not be appropriate to just predict operative complexity on the basis of imaging finding of perinephric infiltration because it could not represent “sticky fat”. Another problem in assessing perinephric fat stranding is its low reproducibility (24), and our study also showed substantial agreement between the two reviewers for perinephric fat stranding.
The development of a scoring system is important to reduce the arbitrariness in determining the surgical approach; however, it is unwise to make the scoring system more complicated by including all relevant factors, because only intuitive and simple scoring systems can be used widely in the urology community (25, 26). Therefore, it is important to consider a proper scoring system by creating a balance between the two values, i.e., more detailed but complex versus simple but less predictive. Sharma et al. showed that the RENAL nephrometry score was associated with the surgical approach, which was intuitively chosen by an experienced surgeon, but the MAP score exhibited no correlation with decision-making between open and robotic-assisted PN (27). In our opinion, in addition to the RENAL nephrometry score, measured PPFT could be reasonably used for predicting operative complexity.
The current study has several limitations. First, it represents a single-institutional, single-surgeon experience, and the number of patients who underwent open PN was small. Second, the presence of APF was not confirmed during surgery. Third, data were collected data over a period of 4 years; it is hypothesized that the surgeon’s surgical skill, which affects ischemia time or operative time, might have improved over time.
In conclusion, the method presented in this study is more reproducible than the method using MAP score. Based on the presented method, increase in PPFT was found to be related to a longer operative time in robotic-assisted PN but not in open PN. Moreover, perinephric fat stranding had little effect on operative complexity in PN.