1. Background
Currently, the trans-Atlantic inter-society consensus (TASC) II classification is being used to assess the severity of peripheral arterial disease (PAD) and establish management guidelines (1). The treatment of choice for TASC A/B lesions is the minimally invasive endovascular therapy. For TASC C/D lesions, surgery has been recommended due to immediate treatment failure of angioplasty as a result of flow-limiting dissection, besides the long-term failure of stents associated with restenosis by intimal hyperplasia (2).
Although paclitaxel-eluting balloons have shown higher primary patency compared to plain old balloon angioplasty (3), bailout stenting in recoil and dissection, especially for long lesions, make the “leave nothing behind” strategy inapplicable in some cases (4, 5). Paclitaxel-eluting stents (PES) are recognized as an alternative treatment by providing a scaffold structure and facilitating the sustained release of antiproliferative drug (6, 7). As described earlier, surgery remains the standard treatment for TASC C/D lesions. However, due to the development of endovascular technologies, including PES, several studies on endovascular therapy for TASC C/D femoropopliteal lesions have reported promising results (8, 9). Endovascular therapy also has several advantages over surgery, such as minimal invasiveness and early recovery after treatment. Future investigations of the promising outcomes and benefits of endovascular therapy, especially PES, can potentially shift the primary treatment preferences for TASC C/D lesions.
Currently, two types of PES, namely, Zilver PTX (Cook Medical, Bloomington, Indiana, USA) and Eluvia (Boston Scientific, Marlborough, Massachusetts, USA), are available for the treatment of femoropopliteal lesions worldwide. While Zilver PTX is a polymer-free nitinol stent that releases more than 98% of the drug within 72 hours (remaining in the blood vessels for 56 days) (10), Eluvia is a fluoropolymer-based stent that releases the drug gradually over 12 months (11). The five-year freedom from clinically driven target lesion revascularization (TLR) is 84.9% for the Zilver PTX PES (12), and the three-year freedom from TLR is 85.3% for Eluvia PES (13). A study in 2018 revealed that the Eluvia stent was not inferior to Zilver PTX stent (14). However, only few studies have compared the results of using both PES types in the real world so far (15, 16).
2. Objectives
This study aimed to assess and compare the real-world outcomes of PES treatment, using either Zilver PTX or Eluvia PES, for patients with TASC type C/D femoropopliteal lesions following suboptimal angioplasty.
3. Patients and Methods
3.1. Patients
The institutional review board of Seoul National University Hospital approved this study (No.: 1910-167-1074). The requirement to obtain patient consent was waived due to the retrospective observational design of this study. From February 2017 to May 2018, patients who underwent endovascular treatment for TASC C/D femoropopliteal lesions with PES (either Zilver PTX or Eluvia) were consecutively included in the study.
The TASC classification was determined based on angiographic findings during the procedure by an interventional radiologist with more than five years of experience. Both de novo and in-stent restenosis cases were included. The patients’ medical records were reviewed, including comorbidities, body mass index (BMI), dialysis history, and history of PAD treatment. Moreover, the symptoms were assessed based on Rutherford classification (categories 0 to 6) (17). As baseline examinations, the ankle-brachial index (ABI) and computed tomography (CT) angiography were applied. Patients with ABI > 1.4 were not included in the analysis, because it represented medial arterial calcification, not pressure or flow (18).
On CT angiography, the degree of calcification was graded as none, mild, moderate, or severe (none, no calcification; mild, eccentric calcification without significant stenosis or obstruction; moderate concentric calcification without significant stenosis or obstruction; and severe concentric calcification with significant stenosis or obstruction). The lesion characteristics, including the grade of stenosis, length, and TASC classification, were assessed based on the diagnostic angiographic findings before endovascular therapy.
3.2. Institutional Protocol
In our institution, not all patients with TASC C/D lesions were treated with endovascular therapy. We discussed the treatment method with a surgeon before making a decision, while considering the patient’s CT angiography and clinical findings. In a multidisciplinary discussion, we decided to perform endovascular therapy first and surgery later if necessary. All interventions were performed via femoral access with either an antegrade or retrograde approach using a 6-Fr sheath. An intra-arterial bolus of heparin (3,000 IU) was injected immediately after the sheath insertion. Diagnostic angiography was first performed to assess TASC classification, immediately followed by endovascular therapy. The target lesion passage was achieved with a guidewire through an intraluminal passage, a subintimal passage, or a retrograde puncture, followed by a through-and-through wire technique.
Pre-dilatation was performed using a plain old balloon, inflated to a diameter that was 1 mm smaller than the reference vessel diameter. The PES was applied for cases of flow-limiting dissection, residual stenosis (> 50%) with repeated prolonged ballooning (> 3 min), or lesions longer than 15 cm with heavy calcification, as the response of angioplasty was expected to be low. PES was used to cover the lesion and the adjacent lesion-free segments; if two or more stents were used, the overlapping portion would be approximately 1 cm. Post-dilatation was carried out after the placement of PES with the same balloon used for Pre-dilatation. The operator selected between Zilver PTX and Eluvia PES randomly without any preferences.
3.3. Definitions
Primary patency (PP) was defined as the improvement of symptoms, freedom from significant restenosis, no recurrence, and no further revascularization. Significant restenosis was defined as symptomatic stenosis with a reduction in the lumen diameter by more than 80% in imaging surveillance. Patients without new or worsening symptoms were considered to have achieved PP. Moreover, freedom from TLR was defined as the absence of any revascularization procedure or surgery in the target lesion. Finally, event-free survival was defined as the absence of complications or events related to the treated limb.
3.4. Follow-up
All patients visited the outpatient clinic at three, six, and 12 months after the procedure, and changes in their symptoms and complications were carefully observed. In patients presenting with new or worsening symptoms, imaging surveillance was performed using duplex ultrasound (US) or CT angiography. If there was no recurrence of symptoms after the treatment, it was assumed that significant restenosis had not occurred, and the patient was observed without testing for restenosis. Even during restenosis, in the absence of symptoms, there was no significant restenosis according to the definition.
The ABI was also measured at six and 12 months after the procedure. All patients received lifelong aspirin (100 mg/day) and clopidogrel (75 mg/day) six weeks after the procedure. In case of in-stent restenosis or occlusion, the interval between the procedure and further treatment was recorded. All complications were classified according to the 2017 Society of Interventional Radiology (SIR) scale (19).
3.5. Statistical Analysis
The normal distribution of data was assessed using Kolmogorov-Smirnov test. Depending on the normality of data, parametric or nonparametric tests were performed for data analysis. The one-year PP rate, freedom from TLR, and event-free survival were analyzed based on the 12-month clinical, imaging, and follow-up results by plotting Kaplan-Meier survival curves. Loss to follow-up and death were considered as censored data. The survival curves were compared using a log-rank test. Continuous variables were summarized as mean and standard deviation (SD) and compared using independent samples t-test, Wilcoxon rank-sum test, and analysis of variance (ANOVA). Besides, paired samples t-test was also used to compare changes in ABI. Categorical variables were also compared using Fisher’s exact test. P-value less than 0.05 was considered statistically significant. All calculations and analyses were performed in SPSS version 26.0 (IBM, Armonk, NY, USA).
4. Results
4.1. Baseline Characteristics
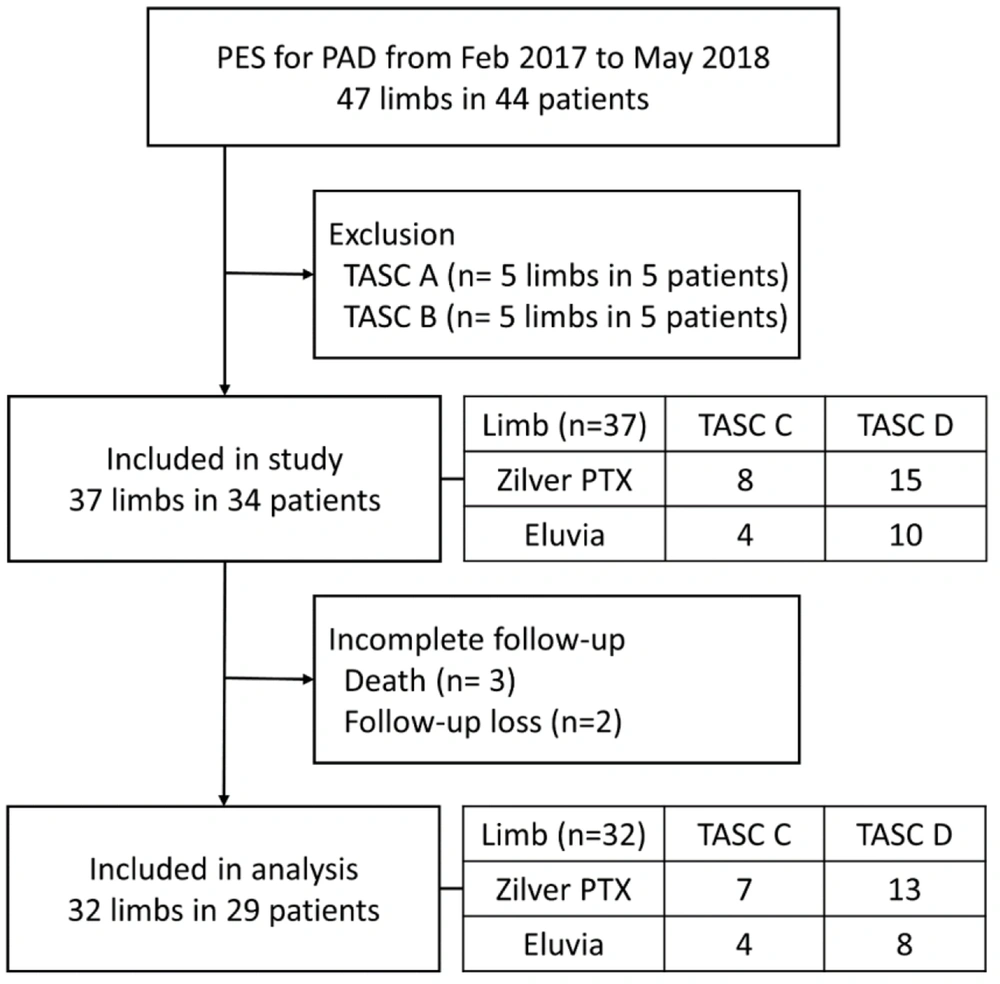
Thirty-seven limbs of 34 patients were examined in this study. Figure 1 presents a flowchart of the patient selection process. The demographic data of the patients are presented in Table 1. Thirty patients were male, and four were female, with the mean age of 71.9 ± 9.10 years (range, 53 - 90 years) and the mean BMI of 23.1 ± 2.80 kg/m2 (range, 18.0 - 29.8 kg/m2). More than half of the patients had comorbidities, including hypertension (n = 21, 62%) and diabetes mellitus (n = 20, 59%). Treatments had been previously performed for 12 (32%) limbs, including seven limbs with stents, two limbs with percutaneous angioplasty, and three limbs with surgery. Rutherford category 3, defined as severe claudication, was identified in 14 (38%) cases, followed by category 5, defined as minor tissue loss, including non-healing ulcers in 10 (27%) patients. The mean duration of symptoms was 28 ± 46.79 months (ranging from two weeks to 20 years).
| TASC C lesions | TASC D lesions | P-value | |
|---|---|---|---|
| Patient (index limb) | 10 (12) | 24 (25) | |
| Age, y | 68.77 ± 10.04 | 73.18 ± 8.58 | 0.203 |
| Male | 9 | 21 | 1.000 |
| BMI | 22.8 ± 2.25 | 23.2 ± 2.93 | 0.677 |
| Chief complaint | 0.567 | ||
| Claudication | 6 | 14 | |
| Pain | 3 | 6 | |
| Non-healing wound | 3 | 5 | |
| Mean period of symptoms, mo | 28.05 ± 23.78 | 27.98 ± 51.37 | 0.997 |
| Rutherford category | 0.930 | ||
| 0 | 0 | 0 | |
| 1 | 1 | 2 | |
| 2 | 1 | 5 | |
| 3 | 4 | 9 | |
| 4 | 2 | 4 | |
| 5 | 4 | 5 | |
| Previous treatment of the index limb | |||
| Stent | 3 | 5 | 1.000 |
| Angioplasty | 2 | 0 | 0.099 |
| Surgery | 1 | 2 | 1.000 |
| Comorbidities | |||
| DM | 7 | 13 | 0.467 |
| HTN | 6 | 15 | 1.000 |
| ESRD (HD) | 4 | 1 | 0.019 |
| CKD (no HD) | 2 | 0 | 0.080 |
| Cerebrovascular accidents | 2 | 5 | 1.000 |
| Coronary artery disease | 3 | 4 | 0.394 |
| Malignancy | 1 | 1 | 0.508 |
Abbreviations: TASC, trans-Atlantic inter-society consensus; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; ESRD, end-stage renal disease; HD, hemodialysis; CKD, chronic kidney disease.
aValues are shown as number or mean ± standard deviation (SD).
The mean preprocedural ABI was 0.47 ± 0.11 (range, 0.25 - 0.77), except for cases with ABI above 1.4. The characteristics of the target lesions are presented in Table 2. Twenty-nine limbs (78%) showed total occlusion, including two cases of stent occlusion. The mean length of the target lesions was 24.6 ± 6.63 cm (range, 9 - 46 cm). Moderate to severe calcifications were detected in 17 (46%) limbs, mild calcifications in 11 (30%) limbs, and no calcification in 9 (24%) limbs. The target lesions were found in the mid-superficial femoral artery (SFA) (86%), proximal SFA (81%), distal SFA (51%), popliteal artery (16%), and common femoral artery (11%). The TASC class D lesions were detected in 25 (68%) limbs and TASC C lesions in 12 (32%) limbs. In 35% of the limbs, all three distal runoff vessels were intact, while 27% had two intact distal runoff vessels, and 38% had one or no intact distal runoff vessel.
| TASC C lesions | TASC D lesions | P-value | |
|---|---|---|---|
| Index limb | 12 | 25 | |
| Occlusion (%) | 5 (42) | 24 (96) | 0.001 |
| Lesion length (cm) | 22.9 ± 5.0 (16 - 33) | 25.5 ± 7.2 (10 - 46) | 0.227 |
| Calcific burden | 0.568 | ||
| None | 2 | 7 | |
| Mild | 2 | 8 | |
| Moderate | 2 | 4 | |
| Severe | 6 | 6 | |
| Occlusion or stenosis > 80% | |||
| CFA | 1 | 3 | |
| Proximal SFA | 8 | 22 | |
| Mid-SFA | 9 | 23 | |
| Distal SFA | 7 | 12 | |
| Popliteal artery | 0 | 6 | |
| Distal runoff | 0.026 | ||
| 0 | 1 | 2 | |
| 1 | 2 | 9 | |
| 2 | 7 | 3 | |
| 3 | 2 | 11 |
Abbreviations: TASC, trans-Atlantic inter-society consensus; CFA, common femoral artery; SFA, superficial femoral artery.
aValues are shown as number or mean ± standard deviation (SD).
4.2. Procedure Outcomes
The guidewire was passed through the occluded segment in an antegrade manner for 34 (92%) limbs, either intraluminally or subintimally. Re-entry devices were used in 47.6% of cases (10 limbs) when re-entry was not possible. The lesion passage was achieved using retrograde punctures in 3 (8%) cases. A mean number of 2.5 ± 0.69 stents (range, 1 - 3) was used in this study, covering 24.3 ± 7.96 cm (range, 6 - 35 cm). For 22 (59%) limbs, three stents were used. A PES with a diameter of 6 mm was used for 35 out of 37 (95%) limbs. A PES of 7 mm was used for the other two limbs (5%). The details of the intervention procedure are summarized in Table 3.
| TASC C lesions | TASC D lesions | P-value | |
|---|---|---|---|
| Type of stent | 0.493 | ||
| Zilver PTX (Cook Medical) | 8 | 15 | |
| Eluvia (Boston Scientific) | 4 | 10 | |
| Number of stents | 0.066 | ||
| 1 | 2 | 2 | |
| 2 | 6 | 5 | |
| 3 | 4 | 18 | |
| Mean | 2.2 ± 0.7 | 2.6 ± 0.6 | |
| Mean total length of stent (cm) | 21.9 ± 8.6 | 25.6 ± 7.5 | 0.191 |
| Target lesion passage | 0.865 | ||
| Intraluminal passage | 5 | 8 | |
| Subintimal passage | 6 | 15 | |
| Outback re-entry device | 4 | 6 | |
| Retrograde puncture | 1 | 2 | |
| SAFARI | 1 | ||
| CART | 1 | ||
| Rendezvous | 1 |
Abbreviations: TASC, trans-Atlantic inter-society consensus; SAFARI, subintimal arterial flossing with antegrade-retrograde intervention; CART, controlled antegrade retrograde subintimal tracking.
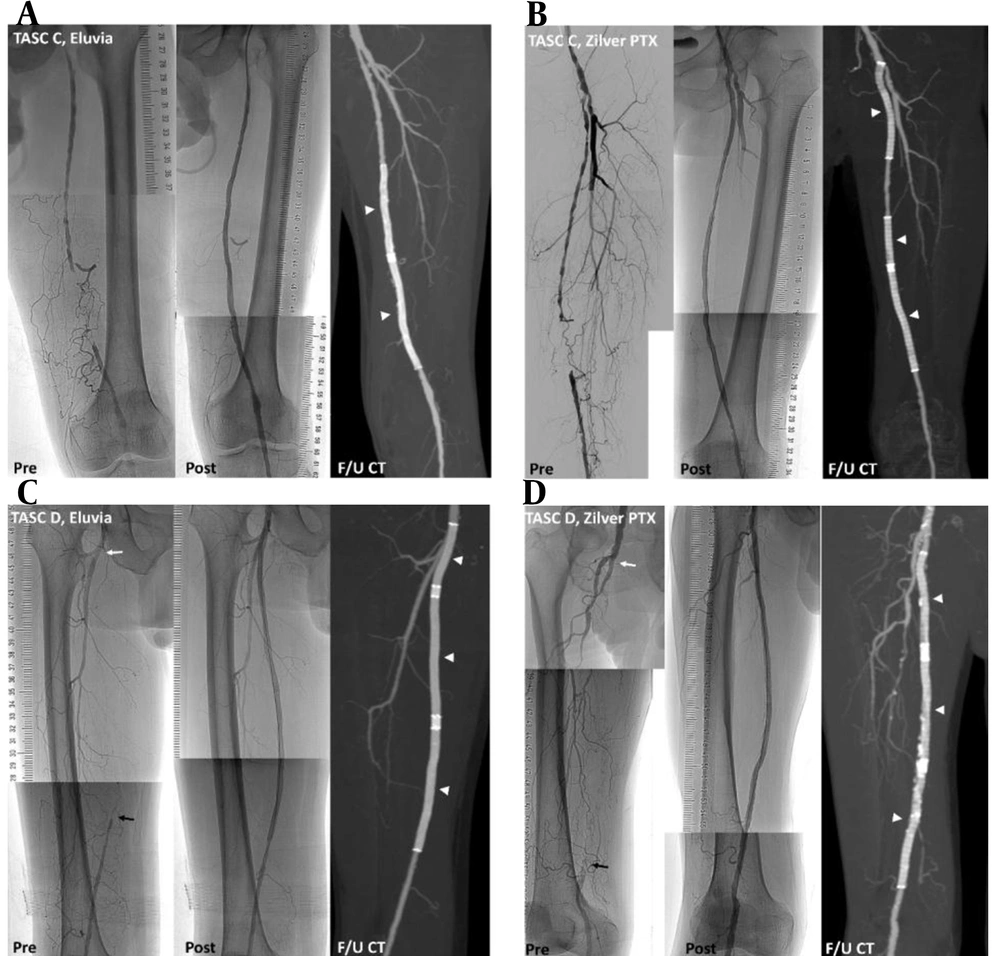
Overall, 56 Zilver PTX stents and 36 Eluvia stents were used in this study; different stents were not combined in the treatment of a single limb. No residual stenosis or insufficient luminal gain was found following post-stent balloon dilatation. Figure 2 presents representative cases of PES for TASC C and TASC D lesions.
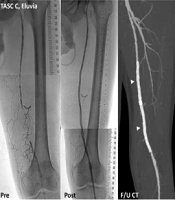
Representative cases successfully treated with paclitaxel-eluting stents (PES). Preprocedural angiography, postprocedural angiography, and follow-up CT scans acquired six months after the procedure (arrow heads) show preserved stent patency. A, A trans-Atlantic inter-society consensus document (TASC) C lesion in a 62-year-old male patient with ulcer and pain in the left big toe, treated with two Eluvia stents. B, A TASC C lesion in a 53-year-old male with 500-m claudication, treated with three Zilver PTX stents. C, A TASC D lesion in a 78-year-old male patient with 50-m claudication and total occlusion from the ostium (white arrow) to the distal superficial femoral artery (black arrow), treated with three Eluvia stents. D, A TASC D lesion in a 75-year-old male patient with 30-m claudication and total occlusion from the ostium (white arrow) to the proximal popliteal artery (black arrow), treated with three Zilver PTX stents.
4.3. One-year Outcomes and Adverse Events
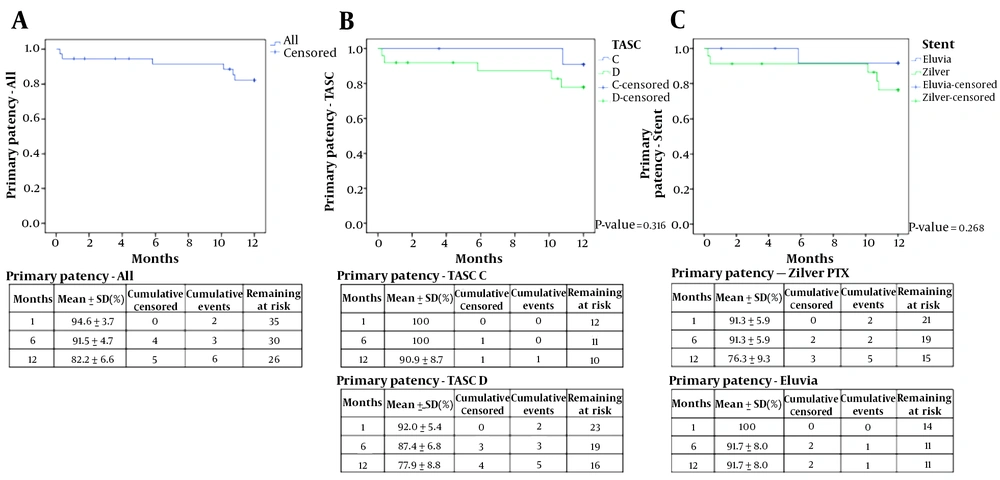
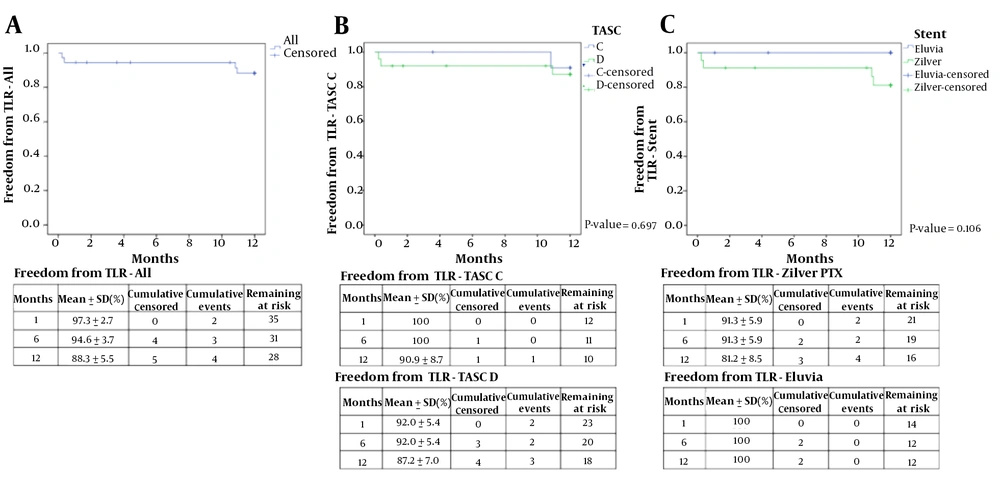
Three patients expired due to pneumonia, stroke, and sudden cardiac arrest, which were not related to the procedure. Since these patients were followed-up for less than a year, their information was incomplete and excluded from the analysis. Finally, 32 limbs of 29 patients were followed-up within a one-year interval from the procedure. Imaging surveillance was performed for 57% of the patients (17 out of 29 patients; CT angiography for 14 patients, and duplex US for three patients). The one-year PP rate was 82.2% based on the Kaplan-Meier survival curve, as shown in Figure 3. Of six limbs whose PP was not maintained, four were detected on CT angiography, one on Doppler US, and one in a physical examination that revealed no dorsalis pedis artery pulse. The one-year freedom from TLR was 88.3%, as shown in Figure 4.
A, Freedom from target lesion revascularization (TLR) at 12 months. B, Subgroup analysis of freedom from TLR at 12 months for TASC C and TASC D lesions. C, Subgroup analysis of freedom from TLR at 12 months for Zilver PTX and Eluvia stents (TASC, trans-Atlantic inter-society consensus document; SD, standard deviation).
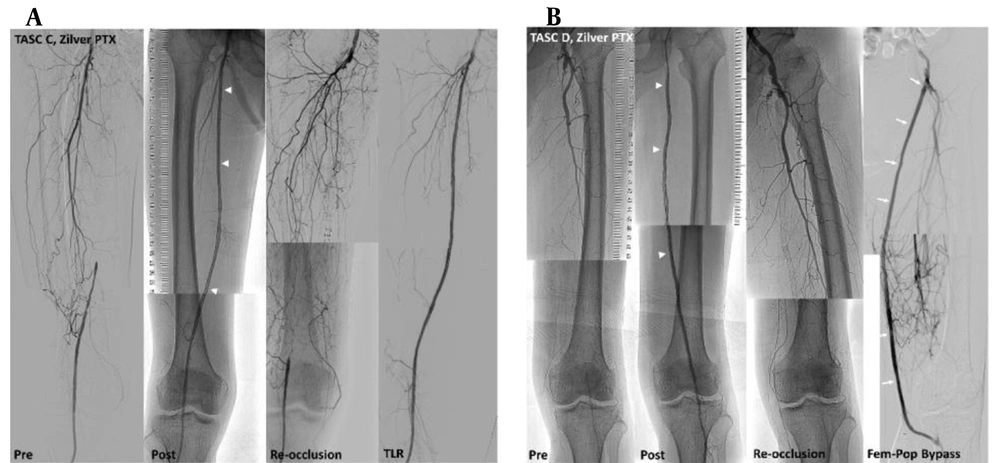
Revascularization of the target lesion was performed in four patients at seven, 11, 325, and 328 days after each procedure. Figure 5 presents two revascularization cases of the target lesion. All four patients were treated with Zilver PTX stents. Since all patients with events underwent revascularization for the treated limbs, the one-year event-free survival rate was the same as the rate of freedom from TLR (88.3%). Besides four TLR patients, one patient underwent amputation of the target limb; however, he was removed from the study, as he expired within a year after the procedure. Also, one patient who received revascularization underwent bypass surgery later.
Cases of revascularization. A, A trans-Atlantic inter-society consensus document (TASC) C lesion in a 61-year-old male patient with 50-m claudication, treated with three Zilver PTX stents (arrowheads). About 10 months after the procedure, the patient complained of leg numbness, and the dorsalis pedis was not palpable. Revascularization was performed using drug-coated balloons and additional Zilver PTX stents. B, A TASC D lesion in an 83-year-old male patient with 20-m claudication and resting pain, treated with three Zilver PTX stents (arrowheads). During hospitalization after the procedure, the patient’s pain worsened, and revascularization for thrombotic total occlusion was performed. After nine months, he complained of toe color change; a femoropopliteal bypass (arrows) was performed for re-occlusion of the femoropopliteal artery.
Based on the subgroup analysis, the one-year PP rates of TASC C and TASC D lesions, regardless of the stent type, were 90.9% and 77.9%, respectively (P = 0.316). Figure 3 shows the one-year PP rates of Zilver PTX and Eluvia stents for TASC C/D lesions (76.3% and 91.7%, respectively) (P = 0.268). The one-year PP rates of Zilver PTX-treated TASC C lesions, Eluvia-treated TASC C lesions, Zilver PTX-treated TASC D lesions, and Eluvia-treated TASC D lesions were 87.5%, 100%, 71.5%, and 85.7%, respectively, without any significant differences. The mean duration of maintaining PP for TASC and TASC D lesions regardless of the stent type was 11.89 ± 0.10 and 10.63 ± 0.67 months, and that with Zilver PTX and Eluvia stent for TASC C/D lesions was 10.76 ± 0.68 and 11.48 ± 0.49 months, respectively.
The rate of one-year freedom from TLR for TASC C and TASC D lesions, regardless of the stent type, was 90.9% and 87.2%, respectively (P = 0.697). Figure 4 shows the one-year freedom from TLR rates of Zilver PTX and Eluvia stents for TASC C/D lesions (81.2% and 100%, respectively) (P = 0.106). The rates of one-year freedom from TLR in Zilver PTX-treated TASC C, Eluvia-treated TASC C, Zilver PTX-treated TASC D, and Eluvia-treated TASC D lesions were 87.5%, 100%, 78.8%, and 100%, respectively, without any significant differences. The mean duration of freedom from TLR for TASC C, TASC D, and Zilver PTX stent was 11.89 ± 0.10, 11.01 ± 0.63, and 10.36 ± 1.02 months, respectively. However, the mean duration of freedom from TLR for the Eluvia stent could not be calculated due to the absence of events.
The event-free survival rates were the same as the rates of freedom from TLR. Overall, TASC C and Eluvia stent showed slightly better results compared to TASC D and Zilver PTX stent, although there was no significant difference. The mean ABI (0.47 ± 0.11; range, 0.25-0.77) improved to 0.89 ± 0.14 (range, 0.54 - 1.08) immediately after the procedure (P < 0.001). At six and 12 months, ABI slightly decreased to 0.84 ± 0.14 (0.59 - 1.05) and 0.82 ± 0.15 (0.56 - 0.99), respectively, without any significant differences (Table 4).
| TASC C lesions | TASC D lesions | P-value | |
|---|---|---|---|
| Preprocedural ABI | 0.52 ± 0.13 (10) | 0.45 ± 0.10 (23) | TASC C/TASC D, P = 0.086 |
| Immediate postprocedural ABI | 0.88 ± 0.08 (7) | 0.89 ± 0.15 (21) | Pre-immediate (all), P < 0.001 |
| Six-month postprocedural ABI | 0.83 ± 0.14 (6) | 0.87 ± 0.14 (15) | Immediate/6 months (all), P = 0.401 |
| Twelve-month postprocedural ABI | 0.88 ± 0.06 (5) | 0.77 ± 0.18 (7) | 6 months/12 months (all), P = 0.519 |
Abbreviations: ABI, ankle-brachial index; TASC, trans-Atlantic inter-society consensus.
aValues are presented as mean ± standard deviation (n).
Adverse events were observed in four limbs, and major adverse events (MAE) requiring treatment occurred in two limbs (2/37, 5.4%). All of these cases had received Zilver PTX stents. They presented with acute thrombotic occlusion and underwent re-intervention within two weeks after the procedure. The other two patients experienced thigh pain and leg edema, which spontaneously improved within a few months.
5. Discussion
In this study, the one-year PP and freedom from TLR rates were 82.2% and 88.3%, respectively, with 78% of TASC C/D lesions showing chronic total occlusion. The average lesion length was 24.6 cm, with moderate and severe calcifications in 46% of cases. The one-year PP and freedom from TLR rates for the Zilver PTX stent were 76.3% and 81.2%, respectively, while the corresponding values for the Eluvia stent were 91.7% and 100%, respectively; however, no significance was found between the stents.
As shown in Table 5, we reviewed the published literature on PES and its clinical outcomes (8, 9, 13-16, 20-23). Among prospective large-scale trials, the STELLA-PTX registry has examined the outcomes of Zilver PTX for TASC C/D femoropopliteal lesions; the one-year PP and freedom from TLR rates were 56.3% and 63.6%, respectively (9). In studies reporting the outcomes of Zilver PTX with a lesion length > 15 cm, the one-year PP rates ranged from 56.3% to 77.6%, and the rate of freedom from TLR ranged from 63.6% to 80.9% (8, 9, 21, 22). Compared to these findings, the corresponding values for the Zilver PTX stents were not below 76.3% and 81.2% in the present study, respectively, suggesting that they are reliable standard techniques and that PES implantation is less operator-dependent.
| No. | Authors | Year | Study title | Inclusion criteria | No. of patients | Type of stent | No. of lesions | LL (cm) | Occlusion (%) | Ca+ (%) | PP (%) | fTLR (%) | MAE (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bosiers et al. (8) | 2013 | Zilver PTX single-arm study | PAD, de novo, and restenotic lesions | 787 | Zilver PTX | 900 | 22.6 | 84 | 75 | 77.6 | 85.4 | 24.3 |
| 2 | Davaine et al. (9) | 2015 | STELLA- PTX for TASC C/D lesions | TASC C/D and de novo lesions | 45 | Zilver PTX | 48 | 25.2 | 56.3 | 63.6 | 11.1 | ||
| 3 | Iida et al. (20) | 2015 | Zephyr | PAD | 690 | Zilver PTX | 831 | 17 | 45 | 65 | 63 | 4.9 | |
| 4 | Bosiers et al. (21) | 2019 | Zilver Pass | TASC C/D and de novo lesions, ≥ 150 mm | 113 | Zilver PTX | 113 | 24.1 | 92 | 74.5 | 80.9 | ||
| 5 | Kichikawa et al. (22) | 2019 | Zilver PTX post-market study in Japan | PAD | 905 | Zilver PTX | 1080 | 14.6 | 42 | 85.5 | 90.6 | ||
| 6 | Muller-Hulsbeck et al. (13) | 2017 | MAJESTIC | PAD, 30-110 mm | 57 | Eluvia | 57 | 7.1 | 46 | 79 | 96.4 | 96.4 | 4 |
| 7 | Gray et al. (14) | 2018 | IMPERIAL | PAD, 30-140 mm | 465 | Zilver PTX | 309 | 8.2 | 31 | 67 | 77.5 | 91.9 | |
| Eluvia | 156 | 8.6 | 30 | 63 | 86.8 | 95.4 | |||||||
| 8 | Bisdas et al. (23) | 2018 | PAD | 62 | Eluvia | 62 | 20 | 79 | 42 | 87 | 87 | 8 | |
| 9 | Soga et al. (15) | 2020 | PAD and de novo, 30 - 200 mm | 48 | Zilver PTX | 12 | 9.7 | 8 | 83.3 | 91.7 | |||
| Eluvia | 36 | 11.6 | 17 | 100 | 100 | ||||||||
| 10 | Soga et al. (16) | 2020 | Japanese patients in the IMPERIAL RCT | PAD, 30-140 mm | 84 | Zilver PTX | 28 | 8.7 | 17.9 | 82.1 | 84.6 | 92.8 | 7.7 |
| Eluvia | 56 | 9.1 | 19.6 | 60.7 | 90.9 | 98.2 | 1.8 | ||||||
| 11 | The present study | TASC C/D, ≥ 150 mm | 34 | Zilver PTX | 23 | 25 | 74 | 57 | 76.3 | 81.2 | 8.7 | ||
| Eluvia | 14 | 24.1 | 86 | 29 | 91.7 | 100 | 0 |
Abbreviations: No., number; PAD, peripheral arterial disease; LL, lesion length; Ca+, calcium level exceeding the moderate level; PP, primary patency; fTLR, freedom of target lesion revascularization; MAE, major adverse event.
Moreover, a study by Bisdas et al. reported the one-year outcomes of Eluvia stents, with a mean lesion length of 20 cm and 79% occlusion (23). The one-year PP rate was 87%, and the freedom from TLR was 87%, which is similar to our results for the Eluvia stents. Recently, Soga et al. published two articles comparing Zilver PTX with Eluvia (15, 16). In a study comparing one-year late lumen loss after PES implantation with a total length of 3 - 20 cm (Eluvia, 36 patients; Zilver PTX, 12 patients) for PAD, the Eluvia stents showed a lower late lumen loss compared to the Zilver PTX stents (0.60 ± 0.8 vs. 1.74 ± 0.89 mm) (P < 0.001) (15). Restenosis was 0% in the Eluvia stents and 16.7% in the Zilver PTX stents, without any significant differences (P = 0.08).
Another study by Soga et al. examined Japanese patients in the randomized IMPERIAL trial (Eluvia, 56 patients; Zilver PTX, 28 patients) on PAD, with a total stent length of 3 - 14 cm (16). The one-year PP rate of Eluvia stent was higher than that of Zilver PTX stents (90.9% vs. 84.6%), and the rate of MAE was much lower for Eluvia stents compared to Zilver PTX stents (1.8% vs. 7.7%); however, there was no significant difference between the stents. In the two mentioned studies by Soga et al., the excellent one-year outcomes of Eluvia stents, as compared to Zilver PTX stents, were confirmed (15, 16).
In the present study, where Eluvia and Zilver PTX stents were implanted into TASC C/D femoropopliteal lesions, the Eluvia performance was superior to that of Zilver PTX stents (one-year PP rate: 91.7% vs. 76.3%). Also, no patients receiving Eluvia stents underwent revascularization, whereas four patients in the Zilver PTX group underwent revascularization (freedom from TLR rate: 100% vs. 81.2%), and all patients with MAE received the Zilver PTX stents (MAE rate: 0% vs. 8.7%). Although there was no significant difference in the current study, our results support and reinforce the results of the two abovementioned studies; however, further research is needed to confirm the statistical significance of our findings.
This study had some limitations. First, long-term follow-up data over three and five years are essential; long-term follow-ups are needed, as previous studies have reported a decrease in the PP and freedom from TLR in three-year follow-ups (12, 13). Second, although the performance of Zilver PTX and Eluvia stents was determined in this study, the sample size was small, and more extensive research is needed. Finally, in this study, cases of in-stent restenosis were also included. However, comparison with de novo cases is essential in a large study population, since the baseline characteristics of de novo and in-stent restenosis can be different.
In conclusion, both types of PES showed promising one-year outcomes for TASC C/D lesions regarding safety and efficacy, without any significant differences. Therefore, they can be considered as an alternative treatment for surgery.