1. Background
The incidence of brain injury in premature infants (BIPI) has been estimated at 10 - 20% (1, 2). Early diagnosis, treatment, and intervention are of paramount importance in BIPI (3). Today, magnetic resonance imaging (MRI), as a multi-directional modality with no radiation damage, is more widely used for newborns and premature infants to study brain development and damage (4, 5). However, most studies have not classified premature infants by birth weight, and there are few reports on premature low-birth-weight (LBW) and very-low-birth-weight (VLBW) infants (1, 3).
2. Objectives
This prospective study aimed to analyze the clinical characteristics of BIPI; to explore the findings of diffusion tensor imaging (DTI) in premature LBW and VLBW infants with BIPI; to examine the value of MRI-based DTI in the evaluation of BIPI; and to seek better methods for early diagnosis of BIPI in premature LBW infants to reduce or alleviate complications in premature infants and improve their quality of life.
3. Patients and Methods
3.1. Study Population
In this prospective study, 31 cases of BIPI, hospitalized in the neonatal intensive care unit (NICU) of Gansu Provincial Maternity and Child Care Hospital (Gansu, China), were examined in 2020 (from 1, 2020 to 11, 2020). The infants’ gestational age was less than 37 weeks, and their birth weight was less than 2500 g. The inclusion criteria for the case group were as follows: (1) abnormal MRI examination of the brain with hemorrhage and white matter damage; (2) severe intrauterine distress, umbilical cord wrapped around the neck, placenta previa, premature rupture of membranes, abruption of the placenta, history of amniotic fluid contamination, hypertension, diabetes, and hyperthyroidism; and (3) clinical manifestations, such as convulsions, increased intracranial pressure, abnormal primitive reflexes, changes in consciousness, and hypotonia.
The BIPI group was divided into two subgroups, according to body weight: LBW group, 1500 g ≤ weight ≤ 2500 g; and VLBW group, 1000 g ≤ weight < 1500 g. Besides, the control group consisted of 20 premature infants, admitted to the hospital with feeding intolerance, scalp hematoma without obvious clinical manifestations of brain injury, and normal MRI results. The Neonatal Behavioral Neurological Assessment (NBNA) was adopted in this study, with a total score of 40. The NBNA score of the control group was > 37 in the one-month follow-up. Both groups of children underwent routine MRI-based DTI examinations at a 37 - 40 weeks corrected gestational age (CGA). Informed consent was obtained from the infants’ guardians (parents) for each examination. The research protocol was approved by the Ethics Committee of Gansu Provincial Maternal and Child Health Hospital (2020[5]).
The clinical information gathered during the study included gender (male/female), gestational age (weeks), birth weight (g), delivery mode (natural labor/cesarean section), twins (yes/no), first delivery (yes/no), one- and five-minute Apgar scores, and maternal age (years). The exclusion criteria in this study were as follows: (1) congenital malformations of the nervous system development; (2) inherited metabolic encephalopathy; (3) chromosomal malformations; (4) hypoglycemic encephalopathy; (5) bilirubin encephalopathy; (6) diseases with a clear etiology; (7) the presence of a congenital heart disease or gastrointestinal malformations; incomplete MRI or follow-up data; and death during the follow-up.
3.2. Brain MRI at Term CGA
In an Avavto 3.0T MR scanner (Siemens, Germany) with a head surface coil, the scanning parameters for different images were as follows: T1WI quiet turbo spin-echo (qTSE) sagittal sequence: (1) repetition time (TR): 2000 ms; (2) echo time (TE): 9 ms; (3) field of view (FOV): 180 mm; (4) slice thickness: 3.5 mm; and (5) voxel size: 0.6 × 0.6 × 3.5 mm3; T1WI qTSE dark-fluid TRA sequence: (1) TR: 2000 ms; (2) TE: 9 ms, (3) FOV: 180 mm, and (4) slice thickness: 4 mm; T2WI qTSE TRA sequence: (1) TR: 5110 ms, (2) TE: 143 ms; (3) slice thickness: 4 mm; (4) FOV: 180 mm; and (5) voxel size: 0.5 × 0.5 × 4.0 mm3; and T2WI BLADE dark-fluid TRA sequence: (1) TR: 7000 ms, (2) TE: 143 ms, and (3) voxel size: 0.8 × 0.8 × 4.0 mm3. Moreover, the scanning parameters for diffusion-weighted imaging (DWI) sequence were as follows: (1) TR: 4800 ms, (2) TE: 84 ms, (3) FOV: 180 mm, (4) slice thickness: 4 mm, and (5) voxel size: 0.7 × 0.7 × 4 mm3. Also, the scanning parameters for the susceptibility-weighted imaging (SWI) T2WI-SWI3D-TRA-p2 sequence were as follows: (1) TR: 27 ms, (2) TE: 20 ms, (3) FOV: 180 mm, (4) slice thickness: 1.7 mm, and (5) voxel size: 0.7 × 0.7 × 1.7 mm3.
3.3. DTI at Term CGA
The Avanto 3.0T MR scanner (Siemens, Germany) with a head surface coil was used for DTI. In DTI, the scanning parameters were as follows: (1) FOV: 220 × 220 mm, (2) slice thickness: 4.0 mm, (3) TR: 3200 ms, (4) TE: 83 ms, (5) number of diffusion directions: 12, and (6) b values: 0 and 1000 s/mm2. Also, the scanning parameters for T1WI 3D (t1_mprage_sag_p2-iso) sequence were as follows: (1) FOV: 180 mm, (2) TR: 2300 ms, (3) TE: 2.38 ms, (4) slice thickness: 0.9 mm, (5) slice oversampling: 12.5%, and (6) voxel size: 0.8 × 0.8 × 0.9 mm3.
For MRI examinations, oral chloral hydrate (30 - 50 mg/kg) was used for sedating the patient at the hospital sedation center. Earplugs and heat preservation were used, while the neonatologist monitored the process. The DTI post-processing was performed on a Siemens workstation, using Syngo Viewer for post-processing the raw data; the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) maps were automatically generated. The central white matter of the frontal and occipital lobes, centrum semiovale, posterior limb of the internal capsule (PLIC), and ventral thalamus were the regions of interest (ROIs); the size of each ROI was 10 ± 2 mm2. To reduce measurement errors, each ROI was calculated three times, and then, the average value was determined. The diagnostic classification of intracranial hemorrhage was based on Papile’s assessment standards (6), and the white matter damage classification criteria were according to a study by Miller (7).
3.4. Data Analysis
Statistical processing of the questionnaire data was performed in Microsoft Excel, and SPSS version 19.0 (IBM, USA) was used for statistical analysis. Shapiro-Wilk test was used for evaluating the normal distribution of data. Differences in measurements between the groups were first compared using independent samples t-test. If normality and homogeneity of variance were satisfied, independent samples t-test was used to compare the groups. If normality and homogeneity of variance were not satisfied, independent-samples Mann-Whitney or Kruskal-Wallis rank-sum test was used. Differences in nominal data were also compared using χ2 test. P-value < 0.05 was considered statistically significant.
4. Results
4.1. Clinical Data of BIPI and Control Groups
In this prospective study, 31 cases of BIPI with LBW and VLBW, including 19 males and 12 females, were examined. There were also 20 normal premature infants in the control group, including nine males and 11 females. There were no significant differences between the two groups in terms of gender (male/female), mode of delivery (natural labor/cesarean section), primiparity (yes/no), gestational age, birth weight, one-minute Apgar score, five-minute Apgar score, and maternal age (Table 1).
| Variables | BIPI group (n = 31) | Control group (n = 20) | P-value |
|---|---|---|---|
| Gender (male) | 19 (61.29) | 9 (45.00) | 0.254 |
| Delivery mode (natural) | 15 (48.39) | 15 (75.00) | 0.059 |
| Primiparity (yes) | 16 (51.61) | 10 (50.00) | 0.910 |
| Gestational age (weeks) | 32.70 ± 2.74 | 31.39 ± 6.38 | 0.466 |
| Birth weight (g) | 1872.58 ± 742.94 | 1876.75 ± 663.85 | 0.678 |
| One-minute Apgar score | 8.10 ± 0.94 | 7.30 ± 1.72 | 0.129 |
| Five-minute Apgar score | 9.03 ± 1.02 | 8.70 ± 1.26 | 0.336 |
| Maternal age (y) | 30.61 ± 4.57 | 30.65 ± 8.12 | 0.581 |
Abbreviation: BIPI, brain injury in premature infants.
a Values are expressed as No. (%) or mean ± SD.
4.2. Comparison of FA and ADC Values Between the BIPI and Control Groups
In the BIPI and control groups, the FA values were 0.110 ± 0.036 and 0.129 ± 0.016 in the central white matter of the frontal lobe (P < 0.001); 0.141 ± 0.039 and 0.164 ± 0.035 in the central white matter of the occipital lobe (P = 0.007); 0.151 ± 0.027 and 0.174 ± 0.037 in the centrum semiovale (P = 0.006); 0.383 ± 0.111 and 0.501 ± 0.065 in the PLIC (P < 0.001); and 0.156 ± 0.033 and 0.182 ± 0.162 in the ventral thalamus (P = 0.011), respectively. Overall, the FA values were significantly different between the two groups (Table 2).
| Variables | BIPI group (n = 31) | Control group (n = 20) | t/z value | P-value |
|---|---|---|---|---|
| Frontal lobe | 0.110 ± 0.036 | 0.129 ± 0.016 | -4.524 | 0.000 |
| Occipital lobe | 0.141 ± 0.039 | 0.164 ± 0.035 | -2.691 | 0.007 |
| Centrum semiovale | 0.151 ± 0.027 | 0.174 ± 0.037 | -2.730 | 0.006 |
| PLIC | 0.383 ± 0.111 | 0.501 ± 0.065 | -3.617 | 0.000 |
| Ventral thalamus | 0.156 ± 0.033 | 0.182 ± 0.162 | -2.537 | 0.011 |
Abbreviations: BIPI, brain injury in premature infants; FA, fractional anisotropy; PLIC, posterior limb of internal capsule.
Moreover, in the BIPI and control groups, the ADC values were 1.863 ± 0.172 and 1.780 ± 0.168 in the central white matter of the frontal lobe (P = 0.040); 1.771 ± 0.118 and 1.670 ± 0.132 in the central white matter of the occipital lobe (P = 0.012); 1.712 ± 0.192 and 1.599 ± 0.162 in the centrum semiovale (P = 0.032); 1.170 ± 0.098 and 1.122 ± 0.139 in the PLIC (P = 0.033); and 1.119 ± 0.087 and 1.057 ± 0.069 in the ventral thalamus (P = 0.009), respectively. The ADC values were significantly different between the two groups (Table 3).
| Variables | BIPI group (× 10-3 mm2/s) | Control group (× 10-3 mm2/s) | t/z value | P-value |
|---|---|---|---|---|
| Frontal lobe | 1.863 ± 0.172 | 1.780 ± 0.168 | -2.055 | 0.040 |
| Occipital lobe | 1.771 ± 0.118 | 1.670 ± 0.132 | -2.508 | 0.012 |
| Centrum semiovale | 1.712 ± 0.192 | 1.599 ± 0.162 | -2.141 | 0.032 |
| PLIC | 1.170 ± 0.098 | 1.122 ± 0.139 | -2.132 | 0.033 |
| Ventral thalamus | 1.119 ± 0.087 | 1.057 ± 0.069 | -2.705 | 0.009 |
Abbreviations: BIPI, brain injury in premature infants; PLIC, posterior limb of internal capsule; ADC, apparent diffusion coefficient.
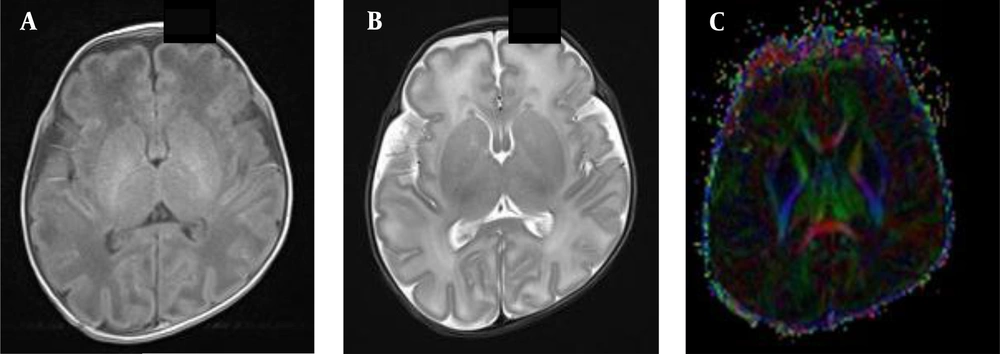
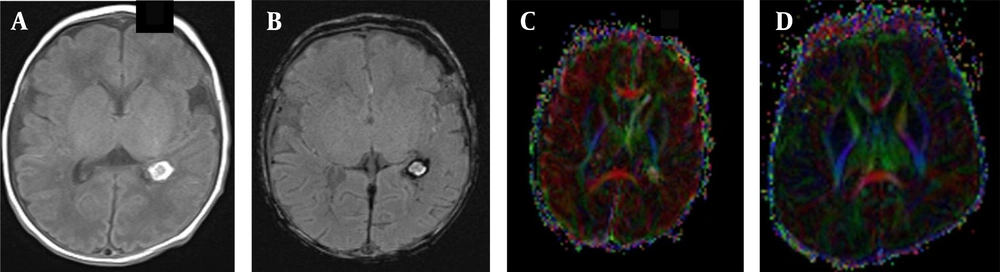
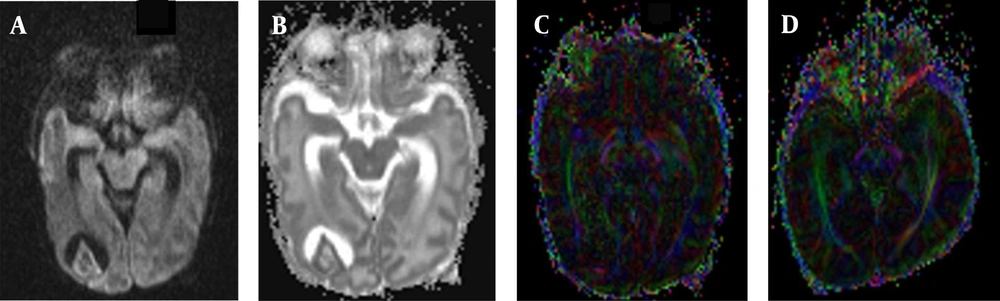
In the BIPI group, the presence of white matter fiber tracts was evident (Figures 1A, B and C). The FA map indicated morphological changes in the white matter fiber tracts of the BIPI group, caused by hemorrhage of the periventricular white matter, resulting in the reduction of white matter, which differs from the control group (Figures 2A, B, C and D). In the BIPI group, the FA map showed the disruption of white matter fiber tracts due to paraventricular leukomalacia (PVL); however, the white matter fiber tracts were normal in the control group (Figures 3A, B, C and D).
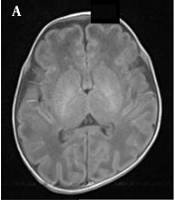
A normal premature female infant born at 33 weeks of gestation, with a birth weight of 1350 g and corrected gestational age of 38 weeks. A, The axial T1WI is normal, and myelination of the posterior limb of the internal capsule (PLIC) is also normal; B, Myelination of the PLIC is normal in the axial T2WI; C, The fractional anisotropy (FA) map shows the white matter fiber tracts in a normal PLIC.
A female newborn with brain injury in premature infants (BIPI) born at 33 weeks of gestation, with a birth weight of 2300 g and corrected gestational age of 38 weeks. A, The axial T1WI shows the hemorrhagic focus of the posterior horn of the left lateral ventricle; B, The axial susceptibility-weighted imaging (SWI) shows left-sided periventricular hemorrhage. C, The fractional anisotropy (FA) map shows that the hindlimb of the left lateral internal capsule is damaged and incomplete and that the white matter fiber bundles on the left side of the ventricle are fewer than those on the right side; D, A normal premature female infant born at 33 weeks of gestation, with a birth weight of 1350 g and corrected gestational age of 38 weeks. The FA map shows that the white matter fiber is normal in premature infants.
A female very-low-birth-weight (VLBW) newborn with brain injury in premature infants (BIPI) born at a gestational age of 29 weeks, with a birth weight of 1469 g and corrected gestational age of 38 weeks. A, The axial diffusion-weighted imaging (DWI) shows the paraventricular leukomalacia (PVL) in the right lateral paraventricular region; B, The apparent diffusion coefficient (ADC) value shows the main hyperintense signal in the PVL; C, The fractional anisotropy (FA) map shows that the white matter fiber bundles in the right lateral ventricle are fewer (incomplete) than those on the left side; D, A normal premature female infant born at 33 weeks of gestation, with a birth weight of 1350 g and corrected gestational age of 38 weeks. The FA map shows that the white matter fiber is normal in premature infants.
4.3. Comparison of FA and ADC Values Between LBW and VLBW Groups with BIPI
In the VLBW and LBW groups, the FA values were 0.104 ± 0.015 and 0.116 ± 0.047 in the central white matter of the frontal lobe (P = 0.782); 0.138 ± 0.040 and 0.144 ± 0.040 in the central white matter of the occipital lobe (P = 0.429); 0.146 ± 0.028 and 0.155 ± 0.027 in the centrum semiovale (P = 0.206); 0.401 ± 0.112 and 0.366 ± 0.112 in the PLIC (P = 0.398); and 0.159 ± 0.033 and 0.153 ± 0.034 in the ventral thalamus (P = 0.638), respectively. The FA values were not significantly different between the two groups (Table 4).
| Variables | VLBW group (n = 15) | LBW group (n = 16) | t/z value | P-value |
|---|---|---|---|---|
| Frontal lobe | 0.104 ± 0.015 | 0.116 ± 0.047 | -0.277 | 0.782 |
| Occipital lobe | 0.138 ± 0.040 | 0.144 ± 0.040 | -0.791 | 0.429 |
| Centrum semiovale | 0.146 ± 0.028 | 0.155 ± 0.027 | -1.265 | 0.206 |
| PLIC | 0.401 ± 0.112 | 0.366 ± 0.112 | 0.858 | 0.398 |
| Ventral thalamus | 0.159 ± 0.033 | 0.153 ± 0.034 | -0.476 | 0.638 |
Abbreviations: FA, fractional anisotropy; VLBW, very low birth weight; LBW, low birth weight; PLIC, posterior limb of internal capsule; ADC, apparent diffusion coefficient.
Moreover, in the VLBW and LBW groups, the ADC values were 1.885 ± 0.121 and 1.842 ± 0.212 in the central white matter of the frontal lobe (P = 0.767); 1.796 ± 0.091 and 1.747 ± 0.138 in the central white matter of the occipital lobe (P = 0.429); 1.719 ± 0.180 and 1.705 ± 0.208 in the centrum semiovale (P = 0.874); 1.172 ± 0.101 and 1.169 ± 0.099 in the PLIC (P = 0.924); and 1.095 ± 0.074 and 1.148 ± 0.034 in the ventral thalamus (P = 0.105), respectively. The ADC values were not significantly different between the two groups (Table 5).
| Variables | VLBW group (× 10-3 mm2/s) (n = 15) | LBW group (× 10-3 mm2/s) (n = 16) | t/z value | P-value |
|---|---|---|---|---|
| Frontal lobe | 1.885 ± 0.121 | 1.842 ± 0.212 | -0.297 | 0.767 |
| Occipital lobe | 1.796 ± 0.091 | 1.747 ± 0.138 | -0.791 | 0.429 |
| Centrum semiovale | 1.719 ± 0.180 | 1.705 ± 0.208 | -0.158 | 0.874 |
| PLIC | 1.172 ± 0.101 | 1.169 ± 0.099 | 0.097 | 0.924 |
| Ventral thalamus | 1.095 ± 0.074 | 1.148 ± 0.034 | -1.621 | 0.105 |
Abbreviations: ADC, apparent diffusion coefficient; VLBW, very low birth weight; LBW, low birth weight; PLIC, posterior limb of internal capsule.
4.4. Comparison of FA and ADC Values Between LBW and VLBW Infants in the Control Group
The results showed that the FA value of PLIC was higher than that of the ventral thalamus, centrum semiovale, central white matter of the occipital lobe, and central white matter of the frontal lobe. The ADC values in the PLIC were lower than those of the ventral thalamus, centrum semiovale, central white matter of the occipital lobe, and central white matter of the frontal lobe (Table 6).
| Variables | FA | ADC (× 10-3 mm2/s) |
|---|---|---|
| Frontal lobe | 0.129 ± 0.016 | 1.780 ± 0.168 |
| Occipital lobe | 0.164 ± 0.035 | 1.670 ± 0.132 |
| Centrum semiovale | 0.174 ± 0.037 | 1.599 ± 0.162 |
| PLIC | 0.501 ± 0.065 | 1.122 ± 0.139 |
| Ventral thalamus | 0.182 ± 0.162 | 1.057 ± 0.069 |
Abbreviations: FA, fractional anisotropy; ADC, apparent diffusion coefficient; PLIC, posterior limb of internal capsule.
5. Discussion
Premature infants with LBW have a higher risk of brain injury due to their immature brain development, resulting in a series of neurological sequelae, poor prognosis, and significant burden on the family and community (2, 8). MRI is an examination method without ionizing radiation. This multi-directional imaging modality is more widely used for neonates and premature infants (3). The present study further explored the correlation between DTI findings and BIPI in LBW and VLBW infants and sought better methods for early diagnosis, treatment, and intervention for BIPI.
DWI is a commonly used imaging technique in MRI. The ADC value is usually measured to describe the diffusion speed of water molecules in the tissue (9). DTI, which was developed based on DWI, can display white matter fiber bundles non-invasively. Generally, there are many anisotropy parameters for quantitative analysis in DTI, with FA being the most common one (10, 11). The FA value denotes the ratio of anisotropy of water molecules to the entire diffusion tensor (range: 0 - 1), which is positively correlated with the integrity of myelin sheath, fiber compactness, and parallelism (12, 13). DTI can completely display the distribution and course of fiber bundles. Regarding the white matter maturity and myelin microstructure integrity, Padilla believes that the FA and ADC values of BIPI are related to brain injury (14, 15).
In the early stage of BIPI, the white matter of premature infants mainly manifests as cytotoxic edema, and diffusion of intracellular water molecules is limited, leading to a decrease in the FA value. In the late stage, when vasogenic edema develops, the cell membrane ruptures, and the amount of free water outside the cell increases relatively, which may cause the FA value to decrease more significantly or below the normal level (13). In premature infants with a white matter damage at term CGA, accompanied by vasogenic edema, DWI shows relatively low signals, and the ADC value is increased, which in turn affects the structure and shape of the white matter fiber bundles and may lead to anisotropic reduction in the FA value. In most studies (13-15), the FA value of BIPI was significantly lower than that of normal infants, and the ADC value was higher in the BIPI group compared to normal infants. However, some scholars (16, 17) believe that the ADC value has no significant correlation with the degree of white matter damage in premature infants.
The present study showed that the FA values in the central white matter of the occipital lobe, central white matter of the frontal lobe, centrum semiovale, PLIC, and ventral thalamus were significantly lower in the BIPI group compared to the control group. The ADCs for the central white matter of the occipital lobe, central white matter of the frontal lobe, centrum semiovale, PLIC, and ventral thalamus were significantly lower in the BIPI group compared to the control group. It seems that the FA value is more statistically significant than the ADC. The results of this study are consistent with those reported by Fukasawa et al. (18-20).
When myelination is delayed or nerve fiber bundles are damaged, water diffusion dyskinesia occurs, the degree of anisotropy is reduced, and the FA value is decreased; it can also manifest as a decrease in the rate of FA increase. Therefore, the degree of FA decline is closely related to brain damage (21, 22), and the FA diagram can reflect the degree of anisotropy through signal strength directly and indicate the speed of tissue water diffusion indirectly. In this study, intra-white matter hemorrhage and PVL caused damage, interruption, and reduction in the brain white matter. The FA chart can clearly show these lesions, which is consistent with the results reported by Zubiaurre-Elorza et al. (23).
Berman et al. (24) Vigneron (25) found that the FA value varies in different white matter areas of premature infants. The FA value differs for the white matter in different parts of the newborn’s brain tissue and gradually increases with an increase in gestational age. This study showed that the FA value of PLIC was higher than that of the ventral thalamus, centrum semiovale, central white matter of the occipital lobe, and central white matter of the frontal lobe. The ADC values were lower in the PLIC compared to the ventral thalamus, centrum semiovale, central white matter of the occipital lobe, and central white matter of the frontal lobe. Therefore, FA and ADC can reflect the brain development of premature infants (26, 27). During the brain development of premature newborns, the ADC of brain tissue gradually decreases with age, while the FA value gradually increases with age. This reflects the maturity of the white matter, which is mainly related to an increase in the concentration of myelin and a decline in the extracellular space and water molecules during axon myelination (25, 28).
In this study, comparison of FA and ADC values in the central white matter of the frontal lobe, central white matter of the occipital lobe, centrum semiovale, PLIC, and ventral thalamus between the LBW and VLBW groups with BIPI showed no significant differences. Previous studies have shown that even after correction at term CGA (29), there are still differences in the structural properties of the white matter between premature and term infants. The FA values of the white matter, striatum, PLIC, external capsule, and corpus callosum in premature infants were still significantly lower than those of term newborns (29, 30). In the current study, premature infants with BIPI were divided into LBW and VLBW groups, according to their weight. However, there was no significant difference in the FA and ADC values between the two groups at term CGA, indicating that weight may not be a highly influential risk factor.
This study had some limitations. First, the number of collected samples was small, and there were certain local restrictions, due to which we could not objectively evaluate the overall brain development in premature infants. Second, some children might have mild brain damage, which was difficult to distinguish on imaging and could be misclassified as brain changes in normal premature infants. To find suitable clinical indicators to determine the severity and prognosis of prematurity in infants, more large-scale studies are needed for further exploration.
In conclusion, this study found that DTI can be used for the quantitative evaluation of BIPI and prediction of its prognosis, which are helpful for the early treatment of patients to improve the neurodevelopment and long-term prognosis of BIPI.