1. Background
Breast cancer currently ranks the first most common female malignant cancer, posing a serious threat to women’s health. Therefore, early diagnosis and timely treatment are essential for all breast cancer patients (1, 2). In recent years, imaging technologies have played an important role in the early diagnosis of breast cancer (3). Magnetic resonance imaging (MRI), as the most sensitive imaging method for breast cancer, can be used to evaluate breast lesions in terms of morphology, blood perfusion, water diffusion, and biochemical metabolism through multiple scanning sequences. However, due to low specificity, lack of microcalcification detection, long duration, and selectivity for patients, it is difficult to apply this method in most primary healthcare units.
Digital breast tomosynthesis (DBT), which can significantly improve the detection rate of breast lesions, has been applied to screen breast cancer since 2011. Breast lesions with different shapes can be clearly visualized in different positions using three-dimensional (3D), multi-layered, projection images (slice thickness: 1 mm) at different angles. Compared to other methods, DBT is more specific and sensitive in the detection of breast lesions, especially dense breast tissues. It is a novel imaging modality which has replaced full-field digital mammography (FFDM) in the diagnosis and screening of breast cancer in some regions (4-7).
2. Objectives
In this study, the diagnostic efficiencies of FFDM, DBT, MRI, FFDM + DBT, and FFDM + MRI for breast cancer were compared to evaluate their diagnostic value.
3. Patients and Methods
3.1. General Data
The clinical data of patients, who underwent pathological examinations in our hospital from January 2019 to December 2020, were retrospectively analyzed. The inclusion criteria were as follows: (1) undergoing DBT, FFDM, and MRI before surgery or biopsy; and (2) having no history of breast surgery, radiotherapy, or chemotherapy before examinations. On the other hand, the exclusion criteria were as follows: (1) unclear images or incomplete data that could not be analyzed; and (2) a history of breast surgery, radiotherapy, or chemotherapy before examinations. All enrolled patients (n = 210) were women aged 26 - 80 years, with an average age of 53 years. Nipple discharge was detected in 32 cases; breast pain was reported in 83 cases; palpable mass was reported in 198 cases; skin dimpling was reported in two cases; and nipple retraction was found in two cases.
3.2. X-ray Examination of Breast Tissue
The patients underwent FFDM with bilateral craniocaudal (CC) and mediolateral oblique (MLO) views of the breasts and DBT with CC and MLO views of the affected breast on a GE Senographe Essential DBT X-ray machine (USA). The imaging process of DBT included nine low X-ray exposures around the breasts, with an X-ray tube at a scanning angle of 25° to obtain a series of low-dose, two-dimensional (2D) images. Subsequently, the images were reconstructed using a computer post-processing software to obtain a series of three-dimensional tomographic images with different slice thicknesses and 2D V-Preview images.
3.3. MRI Examination of Breast Tissue
A GE Discovery MR750 3.0T system (USA) and four-channel, phased-array coils were used for MRI examinations. The prone position with advanced feet was used to make the breasts symmetrical in the coils. The imaging parameters and sequences were as follows: (1) axial T2-IDEAL sequence: repetition time (TR): 6600 ms, echo time (TE): 43 ms, inversion time (TI): 145 ms, field of view (FOV): 33 × 33 cm, slice thickness: 5 mm, layer spacing: 1 mm, and matrix size: 320 × 192 pixels; (2) axial T1-weighted sequence: TR: 560 ms, TE: 10 ms, FOV: 33 × 33 cm, slice thickness: 5 mm, layer spacing: 1 mm, and matrix size: 320 × 224 pixels; (3) axial diffusion-weighted imaging/echo planer imaging sequence: TR: 5600 ms, TE: 69 ms, FOV: 33 × 33 cm, slice thickness: 5 mm, layer spacing: 1 mm, matrix size: 128 × 130 pixels, and b-values: 0 and 800 s/mm2; and (4) axial 3D volume imaging and axial dynamic contrast-enhanced scan: TR: 4.3 ms, TE: 1.6 ms, FOV: 32 × 32 cm, slice thickness: 0.7 mm, and matrix size: 320 × 320 pixels (scanning once before contrast injection and repeated six times 30 seconds after the injection). The contrast agent (gadopentetate dimeglumine) was administered at a dose of 0.2 mL/kg body weight at a flow rate of 2.5 mL/s.
3.4. Imaging Analysis
The FFDM, DBT and MRI images were read by two experienced senior radiologists in a double-blinded manner. The imaging data were divided into five groups (FFDM, DBT, MRI, FFDM + DBT, and FFDM + MRI) and read five times within an interval of at least two weeks. In case of disagreement, another senior radiologist contributed to the discussion to reach a consensus. Breast lesions were diagnosed and evaluated according to the classification criteria of the Breast Imaging-Reporting and Data System (BI-RADS) (2013) (8). The BI-RADS-MRI 4A category or lower indicated benign or negative lesions, while the BI-RADS-MRI 4B category or higher indicated malignant or positive lesions.
3.5. Pathological Diagnosis
The samples were fixed, embedded, and sectioned, and then, immunohistochemical staining and hematoxylin and eosin (H&E) staining were performed. The slices were examined by an experienced senior pathologist to identify the histopathological types. A positive pathological diagnosis indicated a histologically malignant lesion, while a negative diagnosis indicated a histologically benign lesion.
3.6. Statistical Analysis
SPSS Version 26.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Numerical data are expressed as percentage (%), and χ2 test or Fisher's exact test was used to compare the sensitivity, specificity, and accuracy of the five examination methods. The receiver operating characteristic (ROC) curve was also plotted to calculate the area under the ROC curve (AUC). Significant differences were determined using independent t-test. P < 0.05 indicated a statistically significant difference.
4. Results
4.1. Clinical Data
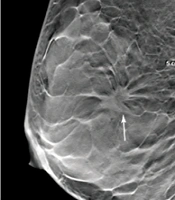
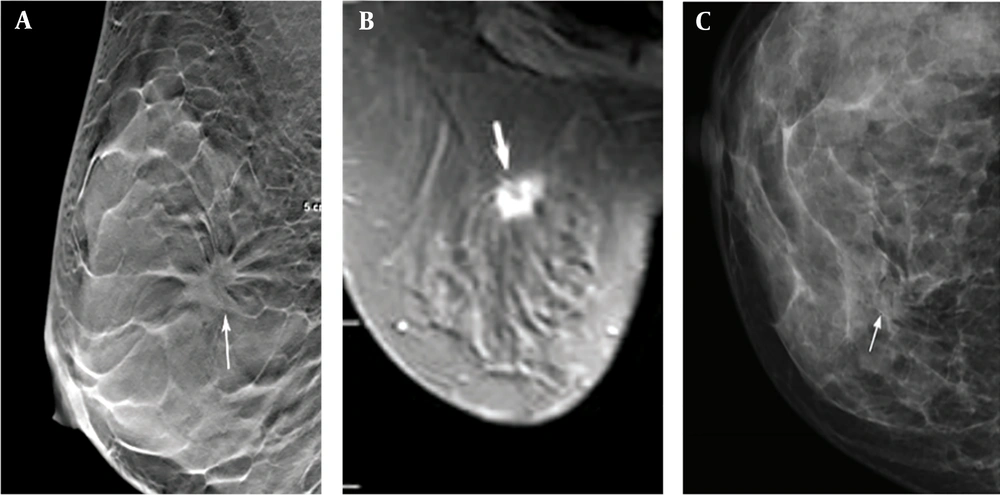
A total of 210 lesions were found in 210 patients, diagnosed by pathological examination; all of the lesions were single breast lesions. Among 105 benign lesions, 48 were found in the left breast and 57 in the right breast. On the other hand, among 105 malignant lesions, 50 were found in the left breast and 55 in the right breast (Table 1). The imaging results of one of the patients are shown in Figure 1.
| Benign and malignant breast lesions | Histopathological type | N |
|---|---|---|
| Benign lesions | Breast fibroadenoma | 58 |
| Adenosis | 28 | |
| Cystic hyperplasia of the breast | 12 | |
| Mammary tuberculosis | 2 | |
| Intraductal papilloma of the breast | 5 | |
| Malignant lesions | Invasive ductal carcinoma of the breast | 83 |
| Invasive lobular carcinoma of the breast | 7 | |
| Ductal carcinoma in situ of the breast | 8 | |
| Mucinous adenocarcinoma of the breast | 5 | |
| Premalignant breast lesions | 2 |
The imaging results of a 45-year-old female patient with a dense, uneven tumor (ACRc) diagnosed as stage II invasive ductal carcinoma. A, The DBT image of the largest layer indicates burrs on the edges of the mass (arrow). B, Fat-suppression T1-weighted MRI image (arrow). C, FFDM image showing overlaps between the mass edges and the mammary gland (arrow). ACR, American College of Radiology; DBT, digital breast tomosynthesis; FFDM, full-field digital mammography; MRI, magnetic resonance imaging.
4.2. Imaging and Pathological Results of Examination Methods
The diagnostic efficiencies of the five examination methods in this study were analyzed for breast cancer, with pathological diagnosis as the gold standard. Among 210 patients, 105 cases had positive pathological findings, while 105 cases had negative pathological findings. Based on FFDM, there were 116 cases with positive findings and 94 cases with negative findings. Based on DBT, there were 110 cases with positive findings and 100 cases with negative findings. . Moreover, based on FFDM + DBT, there were 110 cases with positive findings and 100 cases with negative findings. Also, based on MRI, there were 127 cases with positive findings and 83 cases with negative findings. According to FFDM + MRI, there were 120 cases with positive findings and 90 cases with negative findings. In terms of the density of mammary glands in 210 patients according to the American College of Radiology (ACR), four fatty type (ACRa), 14 scattered fibroglandular (ACRb), 174 dense and uneven (ACRc), and 18 extremely dense (ACRd) lesions were detected (Table 2).
| Methods | Sensitivity | Specificity | Accuracy | Positive predictive value | Negative predictive value | Youden's index | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| FFDM | 82.86 (87/105) | 76.19 (80/105) | 78.57 (165/210) | 75.00 (87/116) | 82.98 (78/94) | 51.83 | 0.734 (0.723-0.845) |
| DBT | 90.48 (95/105) | 88.57 (93/105) | 89.05 (187/210) | 86.36 (95/110) | 91.00 (91/100) | 72.18 | 0.857 (0.794-0.873) |
| FFDM + DBT | 92.38 (97/105) | 90.48 (95/105) | 90.95 (191/210) | 88.18 (97/110) | 93.00 (93/100) | 76.45 | 0.865 (0.801-0.881) |
| MRI | 100.00 (105/105) | 80.95 (85/105) | 91.90 (193/210) | 82.68 (105/127) | 100.00 (83/83) | 77.94 | 0.883 (0.846-0.912) |
| FFDM + MRI | 100.00 (105/105) | 86.67 (91/105) | 95.24 (200/210) | 87.50 (105/120) | 100.00 (90/90) | 83.45 | 0.924 (0.876-0.986) |
Abbreviations: AUC, area under curve; CI, confidence interval; DBT, digital breast tomosynthesis; FFDM: full-field digital mammography; MRI, magnetic resonance imaging.
4.3. Diagnostic Efficiencies of Five Examination Methods for Breast Cancer
The specificity, sensitivity, accuracy, negative predictive value, positive predictive value, and Youden's index of the five examination methods were compared. MRI had a higher diagnostic sensitivity compared to DBT and FFDM; DBT had a higher diagnostic sensitivity compared to FFDM; and DBT had higher specificity and positive predictive value compared to MRI and FFDM. Besides, FFDM + MRI had a higher diagnostic sensitivity compared to FFDM and FFDM + DBT; FFDM + DBT had higher sensitivity compared to FFDM; and FFDM + DBT had a higher positive predictive value compared to FFDM + MRI and FFDM.
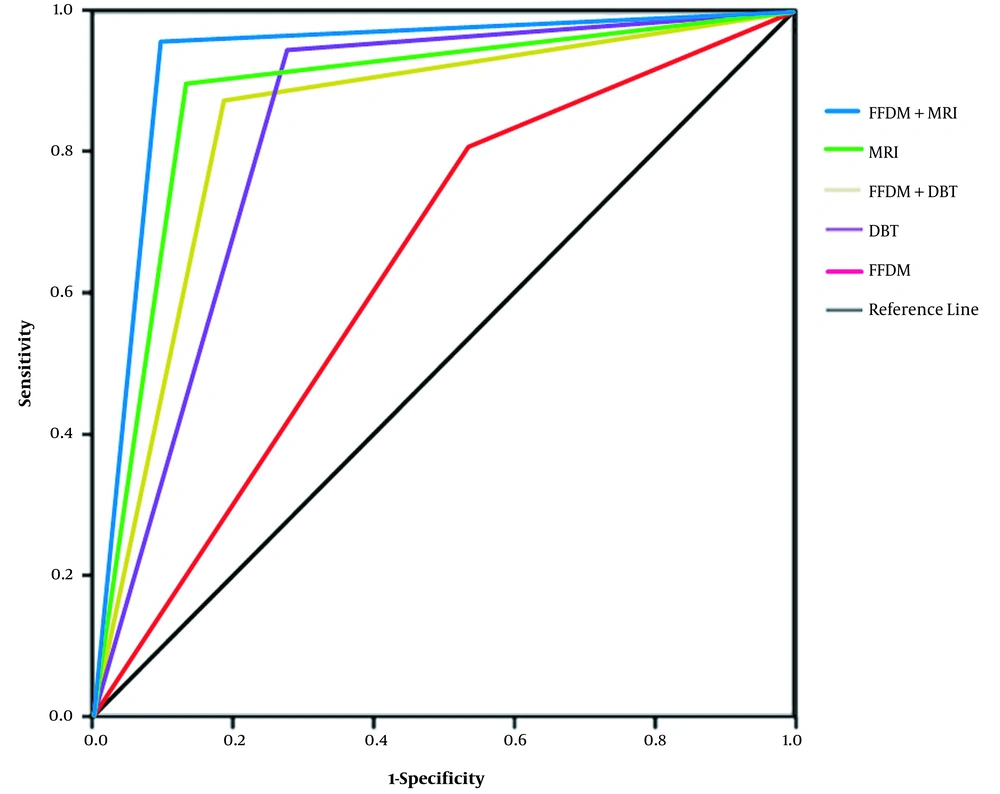
With pathological diagnosis as the gold standard, the diagnostic efficiencies of the five examination methods were analyzed by the ROC curve analysis (Figure 2). The descending order of AUCs for the five examination methods was as follows: FFDM + MRI (AUC, 0.924) > MRI (AUC, 0.883) > FFDM + DBT (AUC, 0.865) > DBT (AUC, 0.857) > FFDM (AUC, 0.734). The diagnostic efficiency of DBT, MRI, FFDM + DBT, and FFDM + MRI was significantly higher than that of FFDM (P < 0.05), while the former four methods had similar AUCs (P > 0.05).
5. Discussion
FFDM is currently recognized as an optimal imaging technology to detect breast tumors. However, the visibility of lesions decreases due to overlap with glandular tissues, leading to the reduction of diagnostic sensitivity and specificity (9). Traditional mammography provides 2D tomographic images that may not detect signs of high diagnostic value, such as burrs or lobes, and may yield false images of malignant lesions, inevitably increasing the false negative and false positive rates (10).
DBT, as a novel technology that combines traditional tomography with digital image processing, can represent the mammary glands from different angles. The low-dose projection data are reconstructed in a computer post-processing software to acquire mammary gland images at any depth parallel to the detector level (11). Compared to traditional FFDM, DBT avoids the overlap of normal glandular tissues with the lesion in imaging, especially for dense mammary glands, improves the visibility of lesion edges (lobes or burrs), and clearly displays lesions with different shapes, heights, and positions hidden in the fibrous gland; therefore, it increases the detection rate of cancer lesions, as well as the sensitivity and accuracy of diagnosis, while reducing the false positive rate (12).
DBT can clearly display the lesion edges (burrs), suggesting its higher diagnostic accuracy and detection rate for early breast cancer, especially dense breast tissues (13), as confirmed in the present study. DBT combined with FFDM seems to have a higher diagnostic accuracy for breast cancer compared to FFDM alone (14). In the present study, the sensitivity, specificity, and accuracy of FFDM combined with DBT for the diagnosis of breast cancer were 92.38, 90.48, and 90.95%, respectively, which were higher than those reported for FFDM or DBT alone. Although DBT performs better in the detection of lesions and has a higher diagnostic accuracy for breast cancer compared to FFDM, its application is still limited due to increased radiation dose and image readings.
MRI can provide high-resolution images of soft tissues, as well as multi-parameter, multi-sequence, and multi-orientation images. Dynamic contrast-enhanced MRI is recognized as the most adequate imaging technology for the examination of breast tissue (15). However, the results of the present study showed 100% sensitivity and only 80.95% specificity for MRI, probably because of the overlap in the MRI signs of benign and malignant breast lesions or insensitivity of MRI to calcification. Traditional mammography combined with MRI shows a higher diagnostic accuracy for breast cancer, which benefits early diagnosis (16). In the present study, the sensitivity, specificity, and accuracy of FFDM combined with MRI for breast cancer diagnosis were 100.0, 86.67, and 95.24%, respectively, which were higher than those of FFDM or MRI alone. Although MRI seems to have a higher diagnostic efficacy for breast cancer, its application is still limited due to contraindications, long duration, and high cost.
Since BI-RADS category 4 is subdivided into 4A, 4B, and 4C subcategories in the 2013 BI-RADS edition, and only 2 - 10% of 4A lesions are likely to be malignant, in the current study, most lesions were considered to be benign. In this study, BI-RADS-MRI ≥ 4B was defined as malignant lesions to increase pathological relevance. Among five imaging methods, FFDM + MRI had the highest diagnostic efficiency. FFDM + DBT and FFDM + MRI had a markedly higher diagnostic accuracy for breast cancer compared to FFDM.
This study had some limitations. First, the sample size was small (n = 210); therefore, the results need to be confirmed in a larger population. Second, DBT is a novel technology in China, which has not been widely applied; therefore, its advantages remain to be fully validated.
In conclusion, DBT and FFDM + DBT could significantly improve breast cancer diagnosis compared to FFDM; the former methods showed comparable diagnostic efficiencies to MRI and FFDM + MRI. The sensitivity of DBT was lower than that of MRI and higher than that of FFDM, while its specificity and positive predictive value were higher than those of MRI. All of the examination methods had certain diagnostic values, with the highest diagnostic efficiency found in FFDM + MRI. Besides, FFDM + DBT and FFDM + MRI could improve the diagnostic accuracy of breast cancer, which is conducive to early diagnosis.