1. Introduction
46,XY pure gonadal dysgenesis (PGD), also known as Swyer syndrome, was first described by Swyer in 1955. It is a very rare disease, affecting about 1 in 80,000 to 100,000 people (1). The chromosome examination indicates a 46,XY karyotype. Since different degrees of gonadal hypoplasia or degeneration occur in the process of embryonic development due to several factors, abnormal sexual development is probable, and the gonads may be prone to tumors (1-3).
2. Case Presentation
The patient was a 22-year-old female without menstruation during puberty. Her height was 166 cm, her weight was 43 kg, and her body mass index (BMI) was 15.6 kg/m2. There was no positive family history of PGD, and the maternal history of pregnancy was normal. The patient’s chest was funnel-shaped, the breast development was classified as Tanner grade III, the vulva was normal, the pubic hair was classified as Tanner II grade, and the vaginal long was 6 cm. The endocrine examination indicated an increase in the level of follicle-stimulating hormone to 95.0 mIU/mL (normal reference range: follicular phase, 3.5 - 12.5 mIU/mL; ovulatory phase, 1.7 - 7.7 mIU/mL; and menopausal phase, 25.8 - 134.8 mIU/mL). However, the estradiol level decreased to 34.0 pg/mL (normal reference range: follicular phase, 50 - 205 pg/mL; ovulatory phase, 115 - 400 pg/mL; and menopausal phase, 50 - 110 pg/mL). Tumor markers, including alpha-fetoprotein (AFP), carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), and carcinoembryonic antigen (CEA), were found to be normal.
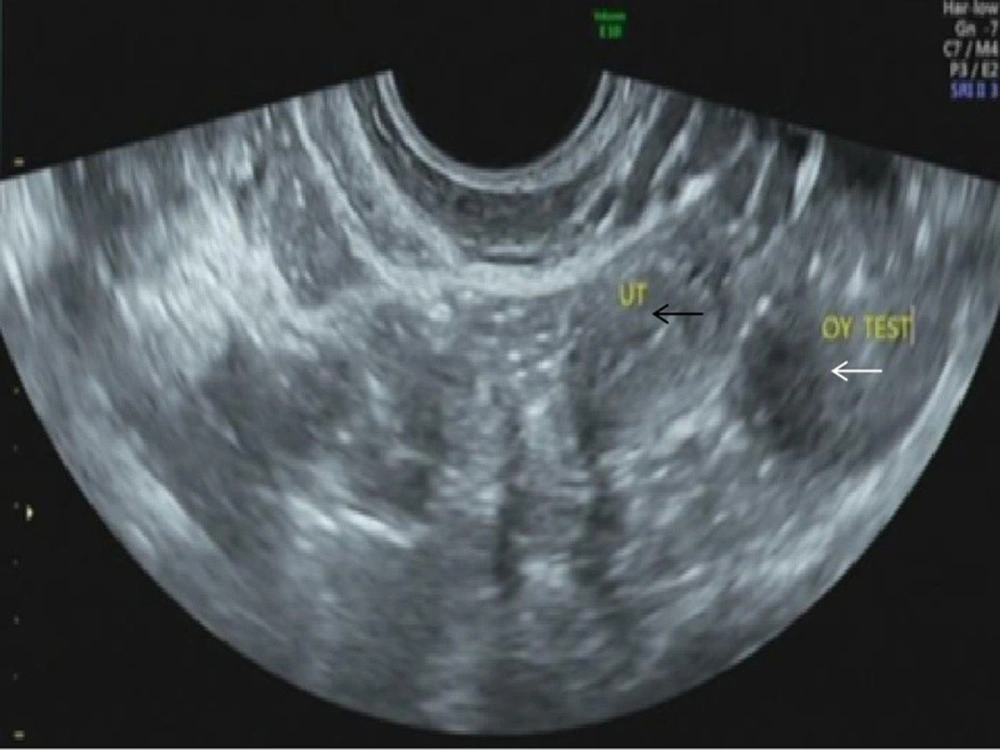
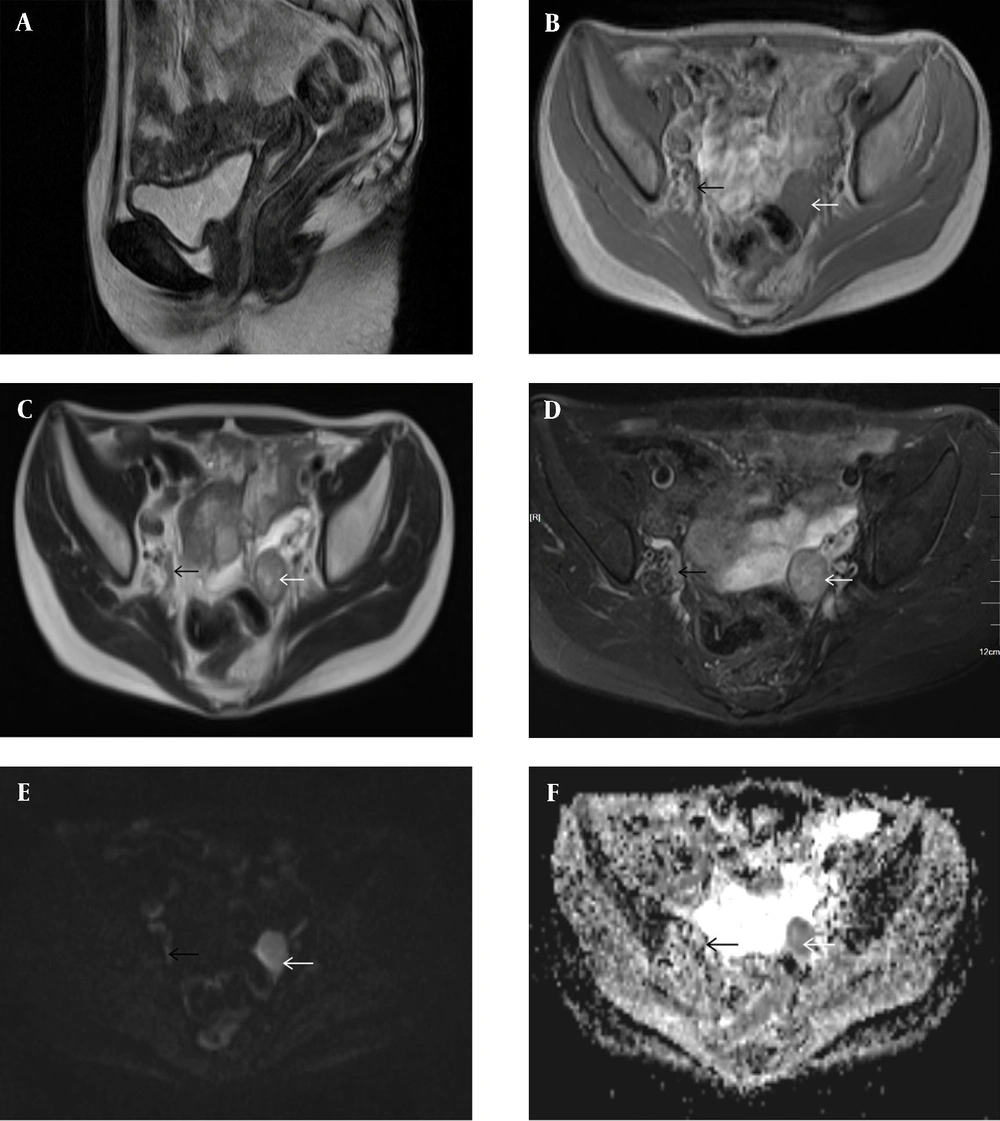
According to dual-energy X-ray absorptiometry, the patient’s bone mineral density was lower than the normal range at her age. The ultrasound revealed that the uterine volume was smaller than normal; a hypoechoic mass was observed in the left adnexal area,considering gonadal tissue with calcification (Figure 1). According to pelvic magnetic resonance imaging (MRI) (Figures 2A-F), the uterine volume was small, and the uterine size was about 1.7 × 1.1 × 2.1 cm; the uterine cavity was not clear, and the cervical canal was visible. Accordingly, an immature uterus was suspected. There was a tumor in the left adnexal region with an approximate size of 2.3 × 2.5 × 3.0 cm, iso-signals on T1-weighted imaging (T1WI), iso-signals and slightly high signals on T2-weighted imaging (T2WI), slightly low signals on diffusion-weighted imaging (DWI) sequences, and low signals on apparent diffusion coefficient (ADC) imaging. A malignant tumor of gonadal tissue was suspected; the gonadal tissue was cord-shaped in the right adnexal area. The chromosome analysis indicated a 46,XY karyotype. Accordingly, a clinical diagnosis of 46,XY PGD was established.
Conventional pelvic MRI with an apparent diffusion coefficient (ADC) map. A, The uterine volume is small (1.7 × 1.1 × 2.1 cm). The uterine cavity is not clear, the cervical canal is visible, and an immature uterus is suspected. B, There is a tumor in the left adnexal region (white arrow), with a size of about 2.3 × 2.5 × 3.0 cm. The T1-weighted image (T1WI) shows iso-signals. C & D, The T2WI image shows iso-signals and slightly high signals (white arrows). E, The diffusion-weighted imaging (DWI) sequence shows a slightly low signal (white arrow). F, The ADC image shows a low signal (white arrow). A malignant tumor of gonadal tissue is detected; the gonadal tissue of the right adnexal area is cord-shaped (black arrows).
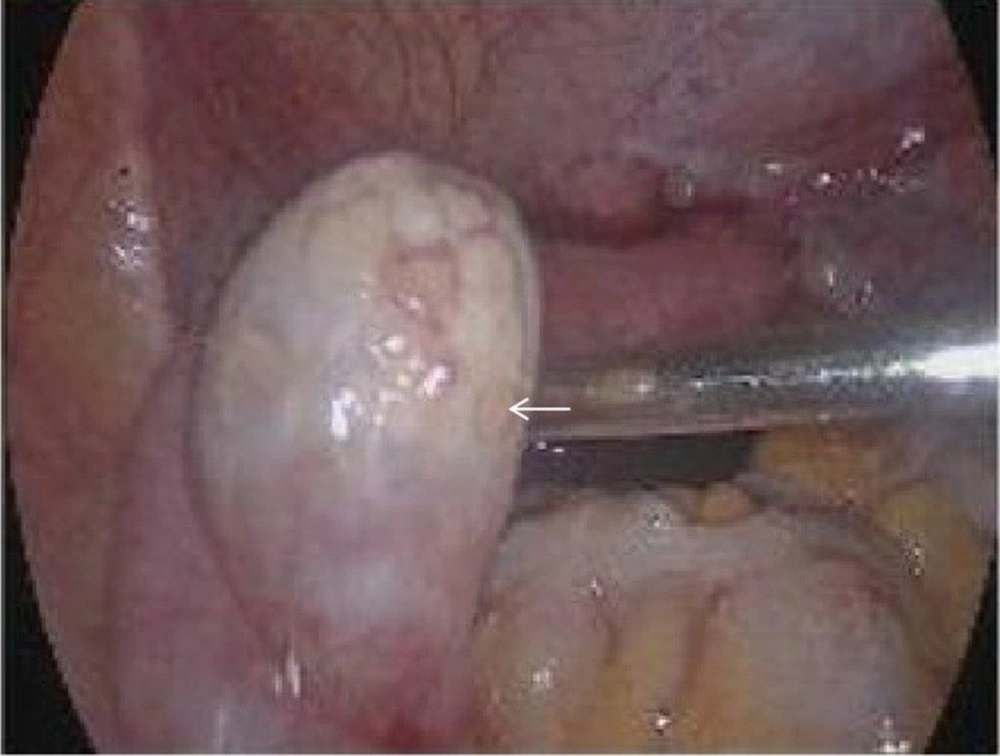
We explained the condition to the patient and informed her of the risk of tumor. With the patient’s consent, bilateral laparoscopic gonadectomy and pathological examination were carried out. The uterus seen during surgery was significantly smaller than normal. The diameter of the left gonad tissue was about 3 cm, and the diameter of the right gonad tissue was about 0.5 cm (Figure 3). The postoperative pathological examination of gross specimens indicated (Figure 4) a grayish-yellow and gray-red left gonadal tissue with solid and firm sections. Also, the right gonadal tissue was gray-yellow and gray-red in color, with gray-yellow and slightly hard sections.
Microscopically (Figure 5), the tumor cells were nesting, and the surrounding fibrous connective tissue was separated. The small round cavity contained an amorphous transparent substance, and Crushed stone-like calcification could be seen. There were two types of tumor cells, including primordial germ cells, which were round with a transparent or slightly granular cytoplasm, a large, round, and vacuolar nucleus, an obvious nucleolus, and mitosis of 5 HPF, and immature Sertoli cells and granulosa cells, which were small and round or oval in shape, with an oval and deeply stained nucleus; also, there was no mitosis-like infiltration of lymphocytes in the fibrous septum around the tumor cell nest.
According to the postoperative pathological examination, a gonadoblastoma with an asexual cell tumor was detected in the left gonadal tissue. The results of immunohistochemistry indicated partially positive Cytokeratin (CKP), negative endomysial autoantibodies (EmA), negative glycine (Gly-3), negative CR, positive CD117, positive SALL4, focally positive α-inhibin, positive Placental alkaline pkosphatase (PLAP), positive podoplanin (D2-40), and weakly positive WT1. The patient was regularly followed-up for two years after surgery. There was no tumor recurrence, the patient’s general condition was good, and the patient’s follow-up was still under way.
3. Discussion
PGD, which is also known as Swyer syndrome, may be sporadic or genetic. The chromosome examination indicated a 46,XY karyotype. Generally, the main clinical manifestations of PGD include primary amenorrhea, absence of secondary sexual characteristics in puberty, lack of or sparse pubic or axillary hair, lack of breast development, and immature internal and external genitalia, fallopian tubes, uterus, vagina, and sex cords (1-3); the gonadal cords are prone to tumors. Today, it is generally believed that gonads with Y-chromosome hypoplasia have a higher risk of gonadoblastoma, and the incidence of gonadal tumors in PGD is higher than that of other types of gonadal dysplasia. However, the mechanism of gonadoblastoma in hypoplastic gonads is not fully understood. The gonadoblastoma gene (GBY, gonadoblastoma locus on the Y chromosome) is the only oncogene located on the human Y chromosome. It plays a physiological role in normal male testicles and represents the role of oncogenes in hypoplastic gonads (3-5).
Gonadoblastoma is a rare gonadal tumor, which often occurs in children and adolescent women. This type of tumor often occurs on both sides and mainly appears on the right side if it is unilateral. However, the present case occurred on the left side. Microscopically, it was mainly characterized by the mixed presence of primordial germ cells and granulosa cell-like, sex cord-interstitial cells (4-6). Generally, germ cells show a clear cytoplasm, an obvious nucleolus, and common mitosis. The sex cord-interstitial components are similar to some immature granulosa cells and Sertoli cells. They are surrounded by small, glassy, eosinophilic, and PAS-positive deposits, which are similar to Call-Exner bodies. Gonadoblastoma can be divided into simple and mixed types. The mixed type refers to other neoplastic components, derived from gonadoblastoma mixed germ cells, such as asexual cell tumor, endodermal sinus tumor, choriocarcinoma, and embryonal carcinoma cells. According to the literature, about 50% of cases are associated with dysgerminoma and 10% with other malignant germ cell tumors, which are easy to miss.
Imaging examinations can provide important information for the evaluation of gonadal dysplasia (7, 8). Gynecologic ultrasonography can detect the echo and blood flow signals of adnexal tumors. When the blood flow is abundant, the possibility of dysgerminoma should be considered. MRI can better indicate the presence, location, size, and extent of gonads and internal genitalia and rule out tumors and related complications through multi-sequence and multi-directional examinations with a good soft tissue resolution (2, 8-11). An abnormal gonadal tissue shows striped and nodular abnormal signals on MRI, most of which are iso-signals on TIWI and slightly low and high signals on T2WI. When a tumor is complicated, the tumor cells are arranged densely, the diffusion of DWI sequence is limited with high signals, and ADC shows low signals.
Evidence suggests that the blood supply to the tumor tissue is not often abundant. Besides, enhancement is not obvious on contrast-enhanced scans. Scattered and small-focus calcifications are also common pathological manifestations of tumors, and calcification can be often seen on ultrasounds. However, MRI is not sensitive to calcifications; therefore, the mass shows a relatively uniform signal on each sequence. Preoperative imaging examination combined with clinical history, chromosomal abnormalities, gonadal dysplasia and other abnormal signs can be considered as gonadal tissue lesions. The results of pelvic MRI examination in our case represent a tumor of a gonadal tissue origin; therefore, chromosome examination is recommended.
The preoperative diagnosis of this disease should be combined with clinical and laboratory endocrine and sex hormone levels, imaging examination, chromosome karyotype and other tests. (11, 12); the final diagnosis is based on pathological results. Also, The differential diagnosis is mainly differentiated from other diseases with abnormal gonadal development. Once diagnosed, bilateral gonads should be resected as soon as possible. Generally, simple gonadoblastoma has a favorable prognosis. If germ cell components have the potential of malignant transformation to asexual cell tumors, the prognosis will improve. The risk of metastasis with other malignant germ cell components, such as endodermal sinus tumor and embryonal carcinoma cells, increases, and the patients have a poor prognosis.
In conclusion, although PGD is a rare disease, a detailed preoperative evaluation of the gonadal size and timely preventive gonadectomy are necessary in patients with gonadal dysplasia. Imaging examinations can provide a suitable basis for the diagnosis of gonadal dysplasia. MRI examinations can clearly represent the size and shape of gonads and tumor, as well as the relationship between the tumor and the surrounding tissue. They also provide important information for the evaluation of preoperative clinical treatments.


![Microscopically (hematoxylin and eosin [H&E] staining, ×10 magnification), there are two types of tumor cells, including round primordial germ cells and immature Sertoli cells and granulosa cells. Microscopically (hematoxylin and eosin [H&E] staining, ×10 magnification), there are two types of tumor cells, including round primordial germ cells and immature Sertoli cells and granulosa cells.](https://services.brieflands.com/cdn/serve/3170b/3959127779ac039f73b5458f5383352d2506f116/iranjradiol-119646-g005-F5-preview.webp)