1. Background
Cryptococcal pneumonia is an opportunistic infection, with the second highest incidence following Aspergillus infection in the lungs of patients with human immunodeficiency virus (HIV). It is classified as an invasive pulmonary fungal infection (IPFI), which can easily invade HIV-infected patients in advanced stages of acquired immune deficiency syndrome (AIDS). The CD4-positive T lymphocyte cell count and HIV viral load are known to directly reflect the immune status and serve as indicators in evaluating the progression of AIDS. Besides, they are recognized as important parameters to monitor the occurrence of opportunistic infections and morbidities of patients (1-3).
According to available evidence on pulmonary cryptococcosis (PC) in AIDS patients, cavity within nodules/masses and solitary nodules are the most frequent computed tomography (CT) findings (4, 5). However, no further investigation has explored the relationship between HIV viral load and lesion morphology. Similarly, the correlation between the CD4 cell count increment and the morphological prognosis of lung lesions has been even more rarely studied.
2. Objectives
This preliminary study aimed to investigate the correlation between CT features and HIV viral load and to determine a cut-off value for CD4 cell count increment in differentiating PC prognosis to assist clinicians and radiologists in diagnosis and evaluation of treatment outcomes and disease progression.
3. Patients and Methods
3.1. Patients
This retrospective, single-center study was approved by the Ethics Review Committee of the institution (Beijing Ditan Hospital, Capital Medical University). The following data were collected from the patients’ medical records available in Beijing Ditan Hospital between June 2017 and June 2021: Age, sex, clinical features, antiretroviral therapy, CD4 cell count, CD8-positive T lymphocyte (CD8) cell count, HIV viral load, chest CT scan findings, and treatment outcomes. Overall, 62 HIV-infected patients were diagnosed with PC. Forty cases had complete clinical and CT data in the three- to six-month follow-up after highly active antiretroviral therapy (HAART). Flow cytometry (BD Corp., USA) was performed to measure the CD4 and CD8 cell count, and a quantitative fluorescent nucleic acid detection reagent (Millipore Corp., USA) was used to measure the HIV viral load in the patients’ peripheral blood.
3.2. Diagnostic Criteria for Cryptococcosis
Definite diagnosis: (1) Positive histopathology, based on the open lung biopsy, percutaneous lung biopsy, and transbronchial biopsy; and (2) positive Cryptococcus culture from the cerebrospinal fluid (CSF) or blood samples.
Possible diagnosis: (1) Positive cryptococcal capsular polysaccharide antigen (CrAg) test in the CSF or serum; (2) typical clinical manifestations (e.g., cough, fever, and expectoration) without other pathogenic bacterial infections; and (3) improvement of patient’s condition and/or pulmonary lesions with anticryptococcal therapy alone. A possible diagnosis could be made when both the first and second criteria or both the first and third criteria were met (6, 7).
3.3. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) HIV positivity; (2) presence of a lesion in the lungs; (3) age above 18 years; and (4) a definite or possible diagnosis of PC. On the other hand, the exclusion criteria were as follows: (1) other concomitant underlying diseases compromising immunity; (2) unclear CT scan findings or not meeting the diagnostic requirements; and (3) non-standard HAART.
3.4. Definitions and Interpretations
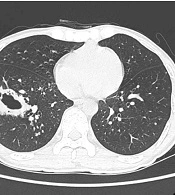
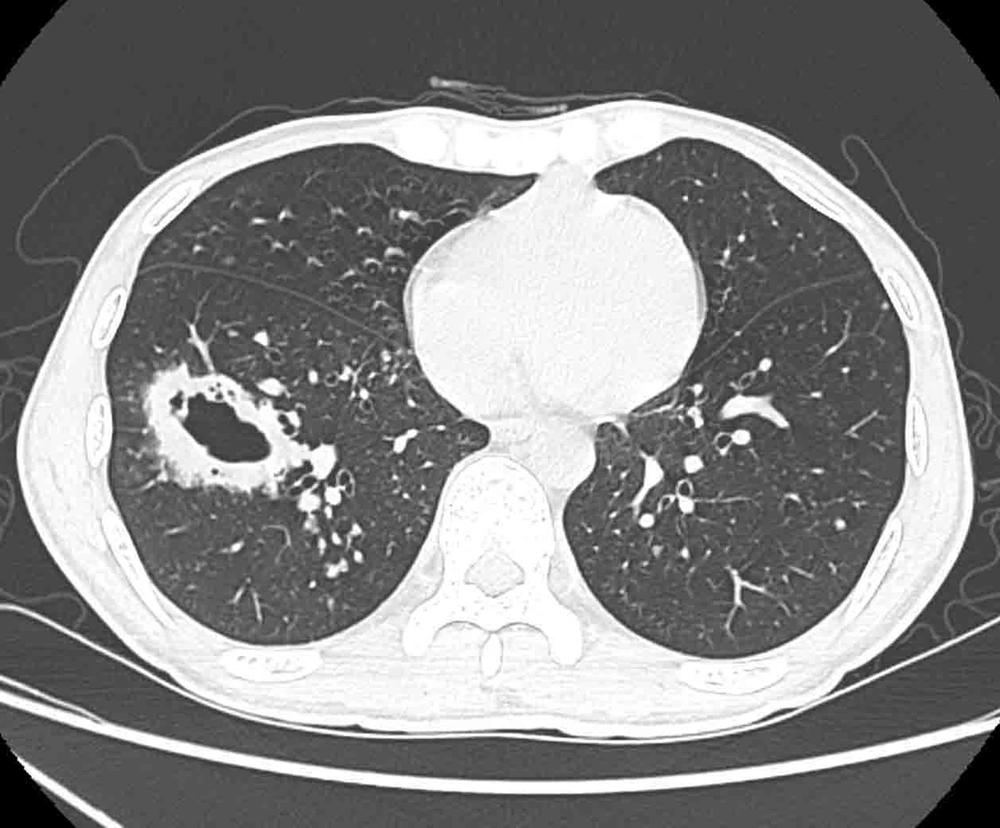
The lung lesions were classified into three patterns: Solid nodules (S), cavitary nodules (CA), and consolidation (C), based on the number and morphology of lesions. Nodular lesions were further subdivided into solitary nodules (SNs), multiple nodules (MNs with < 5 nodules), and miliary nodules (MINs with countless small and randomly distributed nodules). Besides, cavitary lesions were further subdivided into four types, according to the shape and thickness of the hollow wall: Thick-walled cavity (TKC with a cave wall thickness of 4 - 8 mm) (Figure 1); thin-walled cavity (TNC with a cave wall < 3 mm) (Figure 2A); tiny cavity (TC, cavity area < 20% in a nodule-based component) (Figure 2A-C); and cavity in the wall (CW with multiple tiny holes in the cyst wall from partition to partition) (Figure 2A) (8, 9). Consolidation usually manifested with ill-defined margins to normal lung tissue (Figure 2B).
A 27-year-old HIV-infected man with pulmonary cryptococcosis (PC) and a representative thick-walled cavity lesion confirmed by pathology. In the right lower lobe, lesion with irregular thick-walled and line-shaped shadows had an unclear border with the normal lung, while small nodules with sharp borders mixed with ground-glass opacity around the cavity are detected.
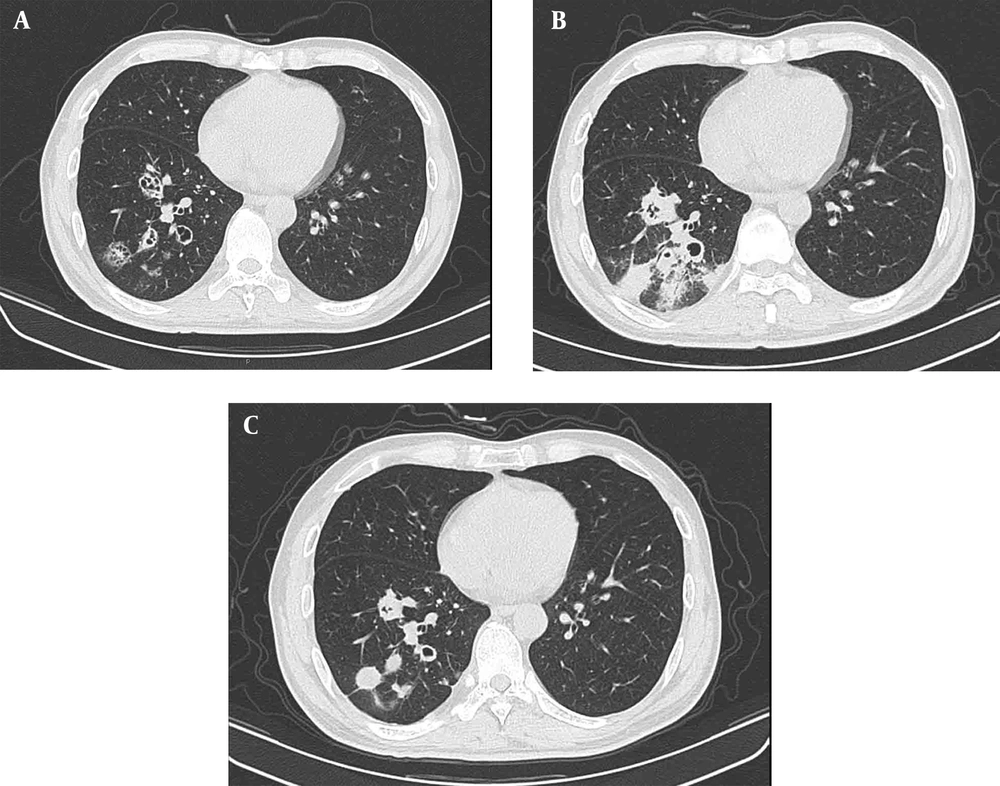
A-C, Pulmonary cryptococcosis (PC) in a 43-year-old man diagnosed with AIDS. CT scan (A) shows several cluster cavities in the right lower lobe, one of which is a cavity in wall (CW) lesion’, while others are thin-walled cavities with a CD4 cell count of 12/µL. Pulmonary lesions progressed to pneumonic infiltrates with ill-defined margins (B) two weeks after highly active antiretroviral therapy (HAART), with a CD4 cell count of 30/µL. In a review CT scan (C) after five months, cavities and consolidation had become solid nodules with clear borders with the normal lung, and the CD4 cell count in peripheral blood increased to 158/µL.
After the onset of HAART, the morphological changes of lung lesions were classified into two categories: Improvement group and progression group. The improvement group included patients in whom the nodules decreased, or the cavities became nodules, or the cavity walls became thinner. On the other hand, the progression group consisted of patients in whom nodules were enlarged and/or increased, or nodules transformed into cavities, or cavity walls became thicker, or ill-defined margin infiltrations were detected. At least one of these conditions suffices to classify a patient in the corresponding group. Two radiologists with more than 10 years of experience in the diagnosis of infectious diseases, who were blind to laboratory indicators, decided about the morphological classification of lesions and progression according to CT findings, including the location, borders, density, and number of lung lesions, as well as pleural effusion and mediastinal lymph nodes.
3.5. Data Analysis
The collected data were analyzed in SPSS Version 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). The clinical indicators are expressed as median and interquartile range (IQR), as well as mean ± standard deviation (SD). The normal distribution of data was examined before statistical tests. All data were analyzed in each group using repeated measures multivariate analysis of variance after Mauchly’s test of sphericity. Moreover, Bonferroni correction test was used for comparison of the three groups. Moreover, a receiver operating characteristic (ROC) curve was plotted to evaluate the performance of diagnostic tests and measure the sensitivity, specificity, accuracy, 95% confidence interval (CI), Youden’s index, and cut-off value for CD4 cell count increment. The inter-observer agreement for the two radiologists was calculated using the intraclass correlation coefficient (ICC). A P-value less than 0.05 was considered statistically significant, and an ICC >0.8 indicated good agreement.
4. Results
4.1. Basic Clinical Characteristics and Radiological Features of the Patients
The clinical characteristics of the patients are presented in Table 1. Of 62 patients, 18 were included in this study with a definite diagnosis, including six patients with a percutaneous lung biopsy, nine patients with a positive Cryptococcus culture from blood samples, and three patients with positive CSF culture; the remaining patients were included with a possible diagnosis. Overall, 59 (95.2%) patients were male, while 3 (4.8%) patients were female, with a median ages of 36.97 ± 11.91 years (range, 19 - 77 years).
| Clinical characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 59 (95.16) |
| Female | 3 (4.84) |
| Age (y), mean ± SD (range) | 36.97 ± 11.91 (19 - 77) |
| Cell count (cells/µL) | P50; IQR (P25, P75) |
| CD4 | 23; (11, 42.5) |
| CD8 | 491; (269.5, 769) |
| CD4/CD8 ratio | 0.05; (0.03, 0.09) |
| Symptoms | |
| Respiratory symptoms | 14 (15.73) |
| Cough | 8 (8.99) |
| Expectoration | 6 (6.74) |
| Chest tightness | 3 (3.37) |
| Dyspnea | 2 (2.25) |
| Neurological symptoms | 31 (34.83) |
| Headache | 24 (26.97) |
| Dizziness | 7 (7.87) |
| Mental disorders | 1 (1.12) |
| Fever or fever combined with other symptoms | 39 (43.82) |
| Other symptoms | 5 (5.61) |
| Cryptococcosis | |
| Only pneumonia | 24 (38.71) |
| Pneumonia + meningitis | 38 (61.29) |
| Comorbidities | |
| PCP | 14 (18.92) |
| CMV | 12 (16.22) |
| TB | 3 (4.05) |
| Syphilis | 5 (6.76) |
| Malignant tumor (cervical cancer) | 1 (1.35) |
| Lesion location | |
| One lobe | 30 (48.4) |
| Two or more lobes | 32 (51.6) |
| Lung involvement | |
| Right lung | 31 (50.0) |
| Left lung | 20 (32.3) |
| Bilateral | 11 (17.7) |
Abbreviations: SD, standard deviation; P50, 50%; IQR, interquartile range; CD4, CD4-positive T lymphocytes; CD8, CD8-positive T lymphocytes; PCP, Pneumocystis carinii pneumonia; CMV, Cytomegalovirus pneumonia; TB, tuberculosis.
Twenty-three out of 62 patients presented to the hospital with multiple symptoms. The main clinical symptoms of 19 patients were pulmonary symptoms; 32 patients first developed neurological symptoms; and 39 patients had fever or fever combined with other symptoms. All cases were complicated with pulmonary complications or other systemic complications, especially cryptococcal meningitis which was detected in the majority of patients (38/62). All lesions were distributed in less than three lobes, including 29 (56.9%) patients with lower lobe involvement, 21 (41.2%) patients with upper lobe involvement, and 1 (1.9%) case of middle lobe involvement. Additionally, there were 14 patients with lymphadenopathy and six patients with pleural effusion.
4.2. Relationship Between Chest CT Findings and HIV Viral Load
The pulmonary abnormalities observed in the initial CT scans and the HIV viral load in the peripheral blood of patients with different types of lesion upon diagnosis are summarized in Table 2. Different types of cavity within nodules/masses (68.4%) were the most common radiological findings. The TNC, TKC, TC, and CW lesions accounted for 32.9%, 13.9%, 16.5%, and 5.1% of radiological cavitation findings, respectively. Solid nodules (27.9%) with well-defined margins or halo signs were the second most common finding. There were also 11 (13.9%) patients with solitary nodules, 7 (8.9%) patients with multiple nodules, and 4 (5.1%) patients with miliary nodules. Another lesion pattern was consolidation, detected in 3.8% of the patients.
| Pulmonary lesions | No. (%) | HIV viral load (copies/mL) | P-value | ||
|---|---|---|---|---|---|
| P50 | IQR (P25, P75) | Within groups a | Between groups b | ||
| Solid nodules | SN vs. MN 0.721; MN vs. MIN 0.024; SN vs. MIN 0.009 | ||||
| Solitary nodules | 11 (13.92) | 2.43E+04 | (3.25E+03 c, 1.30E+05) | ||
| Multiple nodules | 7 (8.86) | 6.78E+04 | (1.75E+04, 2.50E+05) | ||
| Miliary nodules | 4 (5.06) | 3.01E+05 | (1.79E+05, 1.72E+06) | ||
| Count | 22 (27.85) | 5.75E+04 | (7.18E+03, 2.29E+05) | ||
| Cavitation | TC vs. TNC 0.700; TC vs. TKC 0.122; TC vs. CW 0.862; TNC vs. TKC 0.036; TNC vs. CW 0.669; TKC vs. CW 0.356 | S vs. CA 0.932; CA vs. C 0.654; C vs. S 0.588 | |||
| Tiny cavity | 13 (16.46) | 2.44E+04 | (1.15E+04, 1.36E+05) | ||
| Thin-walled cavity | 26 (32.91) | 1.17E+05 | (4.67E+04, 2.57E+05) | ||
| Thick-walled cavity | 11 (13.92) | 1.27E+05 | (8.52E+04, 3.74E+05) | ||
| Cavity in wall | 4 (5.06) | 8.17E+04 | (6.41E+04, 6.17E+05) | ||
| Count | 54 (68.35) | 1.03E+05 | (4.21E+04, 2.29E+05) | ||
| Consolidation | 3 (3.8) | 2.43E+04 | (5.24E+03, 6.10E+05) | - | |
Abbreviations: HIV, human immunodeficiency virus; P50, 50%; IQR, interquartile range; S, solid nodules; CA, cavitation; C, consolidation; SN, solitary nodules; MN, multiple nodules; MIN, miliary nodules; TC, tiny cavity; TNC, thin-walled cavity; TKC, thick-walled cavity; CW, cavity in wall; CT, computed tomography.
a Between groups: Comparisons between three groups of S, C, and CA.
b Within groups: Comparisons between subgroups of patients in each main category of solid, cavitation, and consolidation.
c E+03: Scientific notation, representing E to the third power.
The HIV viral load in peripheral blood was measured at the time of diagnosis before any antiretroviral therapy. There was no significant difference regarding the HIV viral load between different subgroups based on the lesion morphology (S vs. CA, P = 0.932; S vs. C, P = 0.588; and CA vs. C, P = 0.654). In the solid nodule group, the HIV viral load of patients with miliary nodules was significantly higher than that of patients with the other two types of nodules (MINs vs. SNs, P = 0.009; MINs vs. MNs, P = 0.024). In the cavitary group, the HIV viral load of patients with thick-walled cavity lesions was higher than that of patients with thin-walled cavity lesions, and the difference was statistically significant (P = 0.036).
4.3. Correlation Between CD4 Cell Count and Changes in the Lesion Morphology
After HAART, 81.5% of lesions (44 lesions in 40 patients) were classified in the improvement group. Nine nodules (16.7%) decreased, 7 (12.9%) cavity wall nodules became thinner, and 28 (51.9%) cavities became solid nodules. Ten lesions were classified in the progression group, including one enlarged nodule (1.9%), one solitary nodule that developed into multiple nodules (1.9%), two nodules transforming into a cavity (3.7%), three thickened cavity wall nodules (5.6%), and three cases of infiltration with ill-defined margins (6.5%). Forty HIV-infected patients with the median number of CD4 cell count is 23.88 ± 17.51/µL in peripheral blood upon diagnosis, which increased to 96.00 ± 71.55/µL in the 3 - 6-month follow-up after HAART.
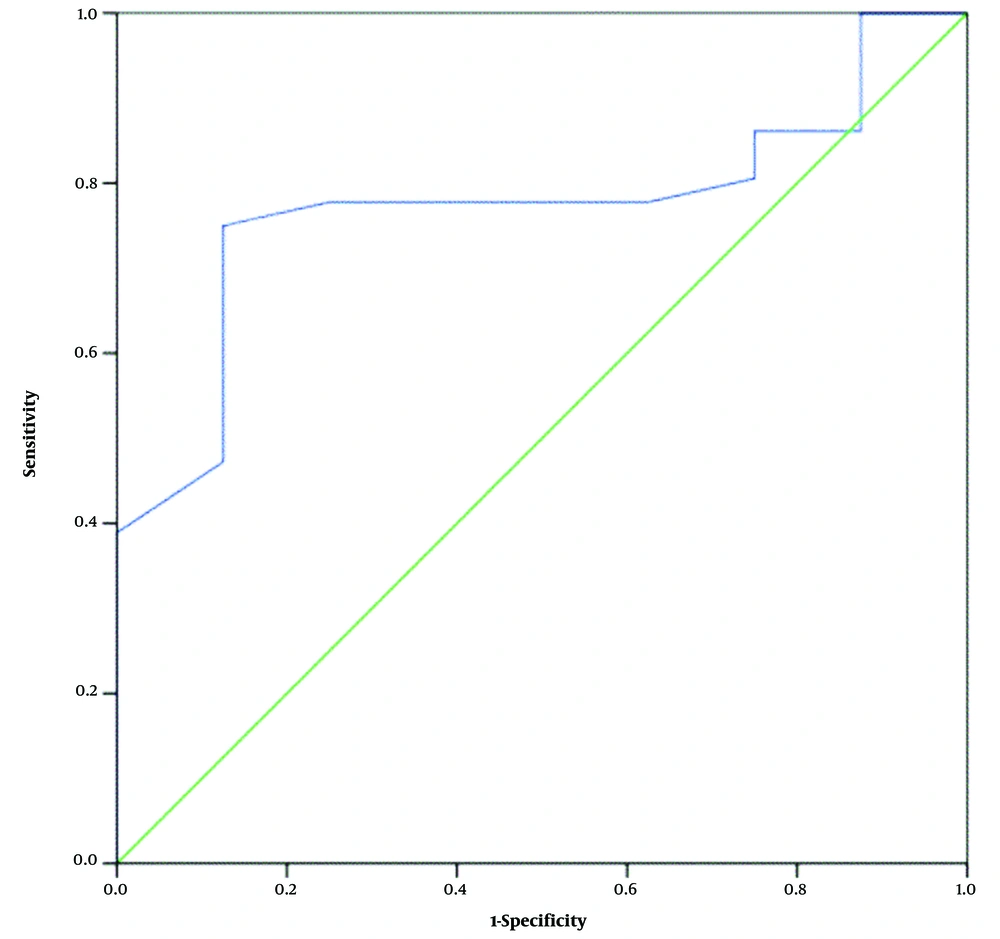
Changes in the CD4 cell count during HAART were also recorded. The mean CD4 cell count increment was 70.6 ± 56/µL in the improvement group and 28.3 ± 19.9/µL in the progression group. There was a significant difference in the mean CD4 cell count increment between the improvement and progression groups (P = 0.001). The cut-off value for the CD4 cell count increment to differentiate the two outcomes (i.e., improvement and progression) was 44/µL, with an AUC of 0.851 (95% CI: 0.81 - 0.88) and sensitivity, specificity, and accuracy of 0.815, 0.714, and 0.764, respectively (Figure 3). Regarding agreement between the two radiologists, the ICC was in the range of 0.824 - 0.871.
5. Discussion
Only few studies on PC have focused on the correlation between CT findings and CD4 cell count or HIV viral load in immunocompromised patients, and most of these studies are case reports or have a small sample size (10, 11). On the other hand, the majority of studies with a larger sample size have concentrated on the clinical and imaging characteristics of Cryptococcus in different immune states (3, 12) or have simply compared the clinical and imaging features of immunocompetent and immunocompromised patients (13). In the present study, the chest CT manifestations of pathologically diagnosed PC were divided into three categories: Nodules, cavities, and consolidation. The CT features were also compared with the HIV viral load of patients at the time of examination, and changes in the lung lesion morphology and CD4 cell count after HAART were analyzed to represent progression or improvement.
Currently, the World Health Organization (WHO) considers the CD4 cell count to be the gold standard for assessing the progression of major opportunistic infections and screening, prophylaxis, and treatment to decrease mortality in patients (14). However, according to the WHO in 2016, when patients are diagnosed with HIV, HAART should be initiated as soon as possible, regardless of the CD4 cell count (15). Meanwhile, detection of HIV load can better indicate an individual’s response to treatment (16); therefore, we measured the HIV viral load to determine its correlation with lung lesions. The viral load decreased rapidly after the onset of HAART, although there was no significant change in the lung lesions. It was suggested that the CD4 cell count increment could reflect the progression or improvement of lung lesions.
The majority of previous studies have divided the CT manifestations of PC into two types: Focal nodular lesions and infiltrating lesions in HIV-negative patient, and solid nodule was the most common lesion type in the lungs (17). In this study, CT findings were classified into three categories of nodules, cavities, and consolidations, according to the number and morphology of nodules. This classification was mainly based on previous research, because with a weakened immune system, the proportion of cavitary lesions and invasive consolidation lesions increases (18). In the current study, the cavitary lesions accounted for 68.34% of all lesions; this rate is much higher than the rates reported in previous studies on HIV-negative patients. It should be noted that if nodules and cavitation are classified in the same category, observations may be deviated or inaccurate. Besides, cavitary and nodular lesions were further classified into different subtypes to further explore the relationship between different morphological lesions and immunodeficiency in the groups.
This study revealed that the HIV viral load of thick-walled cavitary and miliary nodules was higher than that of other nodule types; this difference is mainly attributed to pathological changes after PC inhalation in humans. PC is generally transmitted through the respiratory tract, and fungal spores deposit in the alveoli for colony growth (19). Since a gel-like capsule is wrapped around bacteria in the body, they have no direct contact with tissues, and tissue inflammation is relatively mild in immunocompromised patients; however, a more obvious inflammatory reaction appears when the capsule is lost. In brief, because the immune status of the hosts varies, the morphology of lesions in lungs infected by cryptococcal bacteria is diverse. When the immune status of the host body is good, cryptococcosis can induce delayed-type hypersensitivity after infecting the lungs, forming granulomatous nodules by the way to engulf the cryptococcal bacterium through lung macrophages and tissue cells, as well as fibroblasts and lymphocyte.
Masses can form when nodules fuse or granulomas continue to proliferate. Small focal areas of necrosis occur after the necrotic material is eliminated through the bronchus, and then, various types of cavitary lesions are formed. Consolidation pathologically includes a mixture of cryptococcal hyphae and mucus-denatured connective tissue (20). When the host immunity is impaired, the body’s ability to form fibrotic granulomas decreases, leading to more or larger lung lesions, thickened cavity walls, and consolidation. In contrast, normal host immunity can promote the macrophage phagocytic immunity complex, activate CD8+ T cells to kill target cells, limit the scope of PC infection, and prevent large-scale lung dissemination, manifested in the lungs as reduced lesions, thinned cavity walls, and absorption or limitation of consolidation (21).
Based on the analysis of CD4 increment and the outcomes of pulmonary lesions after HAART, we concluded that a cut-off value of 44/µL for the CD4 increment could best predict the progression or improvement of pulmonary lesions, with a diagnostic sensitivity of 81.5% and specificity of 71.4%. In this study, a follow-up of 3 - 6 months was performed after HAART to observe the CD4 cell count increment and lung lesion outcomes, mainly to reduce the possible influence of immune reconstitution inflammatory syndrome (IRIS) on the outcomes. IRIS is an immunomodulatory response after the onset of HIV reverse transcriptase therapy, reported in nearly 10% of anti-HIV therapies (22, 23). It is associated with various inflammatory factors and chemokines and can manifest as either deterioration of the original opportunistic infection or a new opportunistic infection in other sites, making the imaging process of the lungs complex and variable, as shown in Figure 2A-C.
Since IRIS is a self-limiting disease and also a response to excessive immune reconstitution during therapy, its emergence cannot be considered as disease progression (24). Currently, there are few studies on the occurrence of IRIS in PC. Some studies have reported that IRIS may appear in early stages (i.e., within a few days) after the onset of HAART. While after 6 - 12 months of treatment, the immune status of patients remains stable, the CD4 cell count does not significantly change, although the lung lesions continue to improve. Therefore, in this study, a follow-up of 3 - 6 months was performed to avoid the influence of the two mentioned factors on the results.
In conclusion, based on the present results, the HIV viral load and CD4 cell count in peripheral blood, which reflect the immune status of AIDS patients with PC, are related to the morphology and prognosis of pulmonary lesions, which can be helpful for clinical diagnosis and prognosis evaluation. However, the main limitation of this study was the small number of samples, and our conclusions need to be verified in a larger sample.