1. Background
Currently, remarkable progress has been made in the field of immunoglobulin A nephropathy (IgAN), although its pathogenesis remains unknown (1). Immunoglobulin A nephropathy is recognized as the most common primary glomerular disease around the world (2). A study from China reported that nearly 36.66% of patients with primary glomerular disease were diagnosed with IgAN from 1997 to 2011 (3). One of the pathological features of IgAN is that IgA, with or without other immune complexes, such as IgG and C3, accumulates in the glomerular mesangial area, leading to the extensive proliferation of glomerular mesangial cells and the expansion in the glomerular mesangial matrix to some extent (4, 5). The clinical manifestations of IgAN generally differ. In case of chronic progression, patients with nephropathy experience varying degrees of proteinuria, hematuria, and other symptoms of renal disorders, which can gradually cause renal tissue fibrosis and glomerular filtration rate decline, developing into chronic kidney disease and finally, end-stage renal disease (6, 7).
Common renal examinations include computed tomography (CT) scan and ultrasonography. Despite the advantages of CT scan in clinical diagnosis, disadvantages, such as high cost, radioactive features, and fixed scanning angles, should be addressed (8). Ultrasonography, which is widely used in clinics, is the preferred imaging method in renal examinations, mainly due to advantages, such as being non-radioactive and cost-effective, simple operation, and multi-angle real-time dynamic scanning (9). Conventional technologies have a certain guiding significance for diagnosis, evaluation of curative effects, disease monitoring, and prognosis of renal diseases; however, their sensitivity is limited. Therefore, it is essential to identify a suitable examination with high specificity and sensitivity.
Virtual touch quantification (VTQ) using acoustic radiation force impulse (ARFI) is a type of ARFI technology, which has been widely used for the liver, kidneys, thyroid, and other superficial organs and vascular diseases (10, 11). The VTQ technique is used to measure the shear wave velocity of renal tissues to reflect changes in renal elasticity and hardness following renal dysfunction in IgAN (11). Considering the non-invasive design of this technique, it can be applied many times, which is helpful for clinical diagnosis, evaluation of curative effects, monitoring, and disease prognosis (12). However, the sensitivity and specificity of VTQ in differentiating IgAN patients from healthy controls remain unestablished.
2. Objectives
The present study aimed to explore differences in VTQ values between IgAN and control groups, using routine ultrasound examinations and the VTQ technique. Moreover, the correlation between the VTQ values of renal parenchyma and other parameters, such as conventional ultrasound parameters, renal parenchymal thickness, and Lee’s pathological grade of IgAN was analyzed.
3. Patients and Methods
3.1. Patients and Controls
A total of 29 patients, who were hospitalized in the nephrology department of the Second Affiliated Hospital of Wenzhou Medical University, were randomly selected as the experimental group. The inclusion criteria for IgAN patients were as follows: Diagnosis of IgAN based on renal biopsy and pathological diagnosis; age of 18 years or above; no history of renal disease; and no use of immunosuppressant glucocorticoids before enrollment in the study. The exclusion criteria were as follows: Secondary and primary renal injuries; heart, liver, or lung insufficiency; blood disorders or other serious primary diseases; active tuberculosis; cancer or other consumptive diseases; and incomplete information. Patients with IgAN, including 13 males and 16 females, aged 20 - 57 years, were recruited in this study from August 2018 to February 2019. Histological grading of patients with IgAN was predicted according to Lee et al.’s glomerular grading system (13). Kidney tissue strips, with a size not less than 1.5 cm, were taken from sections with a relatively thick parenchyma under the capsule of the middle-lower pole of the kidney.
The control group consisted of 30 healthy volunteers with normal physical examination indicators during the same period. The controls were randomly selected from the same hospital. The inclusion criteria for the control group were no history of renal diseases, hypertension, diabetes, cardiac diseases, and other chronic diseases. Individuals younger than 18 years and those who refused to sign the informed consent form were excluded from this study. The control group consisted of 15 males and 15 females, aged 19 - 60 years.
The protocols of this study were approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, China. All the participants signed the written informed consent forms.
3.2. Instruments and Inspection Methods
Ultrasonography was performed before treatment for all the participants. The left kidney of each individual recruited in this study was detected via routine ultrasound and VTQ. The participants underwent a two-dimensional ultrasound examination, as well as a VTQ examination in a lateral or prone position. Characteristics, such as the length, width, and thickness of the kidney and parenchymal thickness of the kidney, where the VTQ values were measured, were also recorded. Moreover, the renal volume was calculated according to the ellipsoidal formula (length × width × thickness × π/6). The OXANA2 color Doppler ultrasonic diagnostic apparatus (Siemens, Germany) was used with a 4C1 convex array probe, operating in a frequency range of 1.0 - 4.0 MHz to examine the renal hemodynamics.
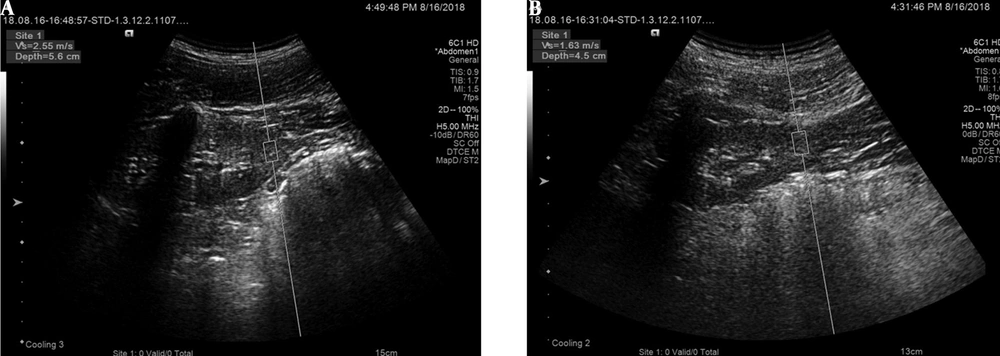
Additionally, resistive index (RI) of interlobular arteries, peak velocity (Vmax) of interlobular arteries, and renal parenchymal VTQ values were measured. The ultrasound beam was perpendicular to the middle-lower capsule of the left kidney and kept fixed as far as possible; the depth of the sampling frame was 8 cm. After adjusting the angle and depth, the VTQ value was measured, and the VTQ image (acquired with the patient holding breath after calm breathing) was stored. The VTQ measurements were performed in the same renal areas as those of Doppler and conventional sonographic measurements. The images in VTQ models are shown in Figure 1. At least six valid measurements of each item were collected from each participant, and the average value was calculated for statistical analysis.
3.3. Intraclass Correlation Coefficient Measurement
The intra-operator and inter-observer reliability studies of VTQ measurements were conducted for the healthy control group. The VTQ testing was performed by the first operator for 30 healthy volunteers. Another operator with extensive experience in ultrasonography performed repeated tests on the same day. The two operators were unaware of each other’s test results. Inter-observer reliability was assessed based on the results of these two tests. Seven days later, the first operator repeated the examinations of each control participant to determine the intra-operator reliability.
3.4. Statistical Analysis
Categorical variables were calculated using chi-square test and expressed as number in the two groups. Shapiro-Wilk test was used to examine the normal distribution of continuous data; if the data had a normal distribution, student’s t-test was used. All continuous variables are expressed as mean ± standard deviation (SD). The relationship between the VTQ values and other clinical characteristics was assessed using univariate linear regression and multiple linear regression analyses. The VTQ values for differentiating IgAN patients from the healthy group were identified using the receiver operating characteristic (ROC) curve analysis. The combined ROC curve was plotted using the VTQ values, renal parenchymal thickness, and peak velocity. There was one single cutoff value in this study, defined as the maximum value of the Youden’s index. This value was measured to determine the sensitivity, specificity, and optimal cutoff points. Additionally, the reliability of VTQ values was evaluated by measuring intraclass correlation coefficient (ICC) at 95% confidence interval (CI) and interpreted as follows: poor, < 0.40; fair to good, 0.40 - 0.74; and excellent, 0.75 - 1. All data were analyzed in SPSS Version 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). A P-value less than 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Parameters
Some important demographic and clinicopathological characteristics of the participants are demonstrated in Table 1 to evaluate their conditions comprehensively. The results revealed that the mean age of IgAN patients was 37.93 ± 9.62 years, and the mean age of the controls was 39.20 ± 11.32 years. There were 13 males in the experimental group, accounting for 44.83% of the total population and 15 males in the control group, accounting for 50% of the total population. No significant differences were found in terms of age or sex between the two groups (P > 0.05 for all).
| Clinical features | IgAN patients (n = 29) | Controls (n = 30) | P-value |
|---|---|---|---|
| Age (y) | 37.93 ± 9.62 | 39.20 ± 11.32 | 0.643 |
| Sex | 0.691 | ||
| Male | 13 | 15 | |
| Female | 16 | 15 | |
| VTQ value (m/s) | 2.14 ± 0.31 | 2.62 ± 0.24 | < 0.001 |
| RI value | 0.68 ± 0.05 | 0.63 ± 0.03 | < 0.001 |
| Renal volume (mL) | 109.99 ± 17.00 | 105.55 ± 16.71 | 0.316 |
| Peak velocity (m/s) | 0.56 ± 0.59 | 0.60 ± 0.58 | 0.005 |
| Renal parenchymal thickness (mm) | 13.67 ± 1.42 | 14.34 ± 1.20 | 0.053 |
| Lee’s grade | |||
| I - II | 15 | - | |
| III - IV | 14 | - |
Abbreviations: IgAN, immunoglobulin A nephropathy; VTQ, virtual touch quantification; RI, resistive index.
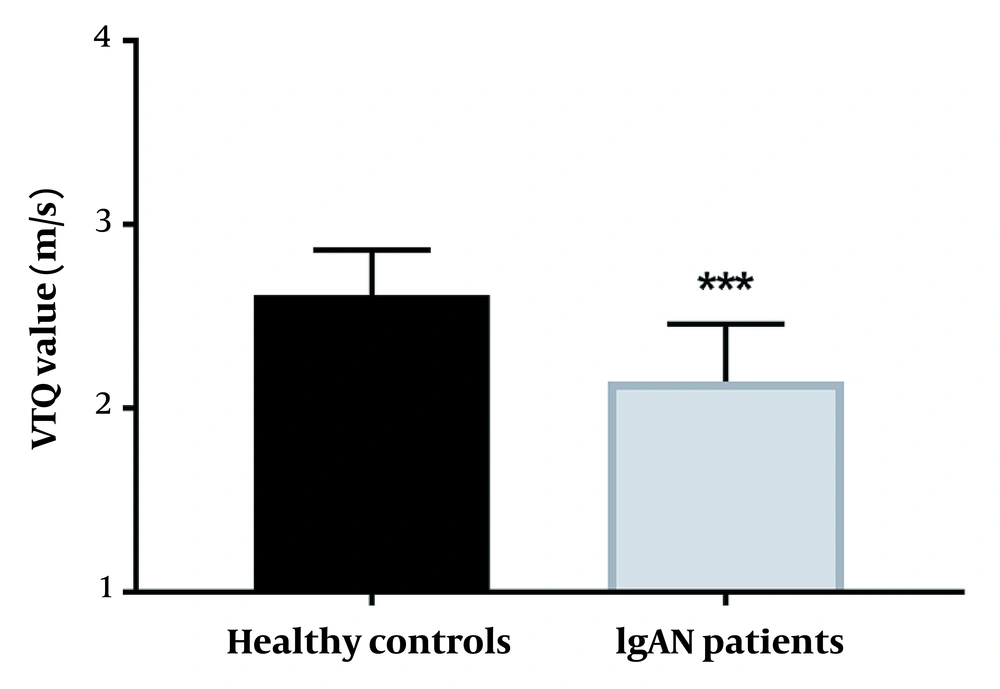
The mean renal volume and renal parenchymal thickness of each group were determined in routine ultrasonic examinations. As shown in Table 1, there was no discrepancy in the renal volume and renal parenchymal thickness between the control and IgAN groups (P > 0.05 for all). The RI value and peak velocity of interlobar arteries were also significantly different between the two groups (Table 1) (P < 0.01). More importantly, the mean VTQ value significantly decreased in IgAN patients (2.26 ± 0.69 m/s) compared to the control group (2.61 ± 0.38 m/s), suggesting the capacity of VTQ to distinguish patients with IgAN from the controls (P < 0.001) (Table 1). According to Figure 2, the average VTQ value reduced in IgA patients compared to the controls (P < 0.001).
4.2. Relationship Between VTQ Values and Clinicopathological Characteristics of IgAN Patients
According to the multiple linear regression analysis, a linear model was established, which included the renal parenchymal VTQ values as the dependent variable and other indicators as independent variables. As shown in Table 2, the renal parenchymal thickness, peak flow velocity of interlobular arteries, and Lee’s grade were correlated with renal parenchymal VTQ values (P < 0.05 for all).
| Items | Univariate linear regression | Multivariate linear regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | t | P-value | Coefficient | SE | t | P-value | |
| Renal parenchymal thickness | 0.199 | 0.032 | 6.244 | < 0.001 | 0.078 | 0.024 | 3.235 | 0.003 |
| Renal volume | 0.008 | 0.003 | 2.561 | 0.016 | - | - | - | - |
| RI value | -2.220 | 1.225 | -1.813 | 0.081 | - | - | - | - |
| Peak velocity | -4.558 | 0.531 | -8.592 | < 0.001 | -1.933 | 0.608 | -3.179 | 0.004 |
| Lee’s grade | -0.258 | 0.031 | -8.254 | < 0.001 | -0.121 | 0.033 | -3.698 | 0.001 |
Abbreviations: SE, standard error; RI, resistive index.
4.3. Analysis of VTQ Values in Differentiating IgA Patients from Healthy Controls
The VTQ value for differentiating patients with IgAN from the healthy group was evaluated based on an ROC curve analysis. As shown in Figure 3A, for the VTQ values, the area under the curve (AUC) was 0.909 (95% CI: 0.836 - 0.983), sensitivity was 0.828, and specificity was 0.867. Based on these findings, the renal parenchymal VTQ values could distinguish patients with IgAN from healthy controls with high sensitivity and specificity. The optimal cutoff value of VTQ was 2.47 m/s, with a maximum Youden’s index value of 0.695. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) are presented in Table 3.
The results of receiver operating characteristic (ROC) curve analysis indicated the diagnostic value of virtual touch quantification (VTQ) values and the combination of renal parenchymal thickness, peak velocity, and VTQ for differentiating patients with immunoglobulin A nephropathy (IgAN) from healthy individuals. A, The VTQ values showed a high diagnostic accuracy in screening IgAN patients from normal people (area under the curve [AUC] = 0.909, sensitivity = 0.828, specificity = 0.867); B, The AUC of combined renal parenchymal thickness, peak velocity, and VTQ values was 0.930, with sensitivity of 0.892 and specificity of 0.867.
| AUC (95% CI) | Cutoff Value | Sensitivity | Specificity | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|
| VTQ value | 0.909 (0.836 - 0.983) | 2.470 | 82.8% | 86.7% | 85.7% | 83.9% | 6.21 | 0.20 |
Abbreviations: AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; VTQ, virtual touch quantification.
As shown in Figure 3B, the AUC for combined renal parenchymal thickness, peak velocity, and VTQ value was 0.930 (95% CI: 0.859 - 0.999), with sensitivity of 0.892 and specificity of 0.867 at a cutoff point of 0.511. At this optimal cutoff value, the Youden’s index was 0.764.
4.4. Inter- and Intra-operator Agreement
For the two operators responsible for VTQ detection, the ICC of intra-operator consistency was 0.720 (95% CI: 0.491 - 0.856, P < 0.001). Regarding the inter-observer consistency, the ICC was 0.675 (95% CI: 0.402 - 0.834, P < 0.001). This finding suggests that VTQ measurements had fair to good reproducibility.
5. Discussion
IgAN is recognized as the most common form of chronic glomerular disease, with complex contributing factors. It leads to end-stage renal disease in 20 - 40% of patients, and the recurrence rate is increasing, which suggests a heavy socioeconomic burden (14). Examinations for renal diseases commonly include morphological analyses, mainly Doppler ultrasound and renal CT to observe the size and shape of the kidneys (15, 16). Considering the limitations of these two methods, the detection rate is not satisfactory, and the identification rate is low. Generally, VTQ is an emerging technique to identify patients by detecting the morphological features. However, there is still limited information on the efficacy of VTQ in the clinical diagnosis of IgAN.
Today, more and more scholars are paying attention to the clinical application of VTQ (17, 18). Some studies discovered that the VTQ values were abnormal in various nephropathies, such as chronic glomerulonephritis, renal proximal tubule damage, and other renal disorders. A study on chronic glomerulonephritis indicated that the mean VTQ value of the right kidney significantly decreased in patients with IgAN compared to the control group (19). Another study by Bob et al. proposed that the VTQ values reduced in patients with chronic kidney disease (20). Although the VTQ values have been measured for these renal diseases, the VTQ values for IgAN remain unclear.
In this study, the mean renal parenchymal VTQ value of patients with IgAN was significantly lower than that of the control group; therefore, the hardness of renal parenchyma decreased due to renal impairment caused by IgAN. Compared to previous findings, the decrease in VTQ values in IgAN was consistent with the corresponding values for other types of nephropathy. The RI value and peak velocity were significantly different between the two groups. However, the renal volume and renal parenchymal thickness were undifferentiated between the IgAN patients and healthy controls. The difference in VTQ values between patients with IgAN and healthy controls suggested that renal parenchymal VTQ values might be used as a predictor.
Given the differences in VTQ values between patients with IgAN and the controls, the association between these values and other characteristics of IgAN was further analyzed. The results of multiple linear regression analysis showed that renal parenchymal thickness, peak flow velocity of interlobular arteries, and Lee et al.’s grade were correlated with renal parenchymal VTQ values, suggesting the possible association of renal parenchymal VTQ values with IgAN (13). The VTQ values were related to the clinicopathological characteristics of many other diseases and applied in clinical examinations. For instance, shear wave velocity detected by VTQ was associated with the mass size and histological grade in women with invasive ductal breast cancer (21). In patients with renal damage, the combination of VTQ value with urinary β2-microglobulin level may be useful in the diagnosis of gouty kidney damage (22). These results indicated that renal parenchymal VTQ values might be associated with the progression of IgAN. Based on the present and previous findings, VTQ might be an effective screening method for disease development.
Extensive growth in research in the last few decades has made it possible to examine IgAN using the VTQ technique. For instance, the VTQ values could differentiate between benign and malignant thyroid lesions by providing quantitative elasticity measurements (23). In advanced chronic liver disease, the VTQ value may be a predictor in clinics, with specificity of 80.8% and sensitivity of 73.7% (24). In severe renal interstitial fibrosis, the AUC for VTQ was 0.954 in the patient group versus the control group, indicating the prognostic value of VTQ in screening renal disorders (25). Inconsistent with the aforementioned observations, the current study showed that the VTQ value could distinguish IgAN patients from healthy individuals.
In the current study, the diagnostic efficacy of the VTQ value was evaluated based on the ROC curve analysis. The results indicated that the renal parenchymal VTQ value might play an essential role in distinguishing IgAN patients from healthy individuals, with high sensitivity and specificity. A previous study reported that the VTQ level was 2.69 ± 0.72 m/s in the right kidney and 2.48 ± 0.73 m/s in the left kidney, which exceeded the VTQ value (2.26 ± 0.69) in IgAN patients (19). In glomerulonephritis, the VTQ value of the parenchyma was 1.55 m/s, while in this study, the VTQ value was 2.26 m/s in IgAN patients; the significant difference between the groups suggests that the VTQ value could distinguish IgAN from glomerulonephritis (26). Accordingly, the VTQ value can be useful in differentiating IgAN from other renal diseases.
The present study had a major limitation. Based on the study design, enrollment of the participants was not useful for the assessment of VTQ efficacy in differentiating IgAN from other renal parenchymal disorders, since IgAN was compared with healthy individuals. Future research needs to compare other glomerular and renal parenchymal diseases with IgAN. Other limitations of this study include the small sample size and lack of comparison of the diagnostic performance of VTQ with conventional ultrasonography and Doppler ultrasonography.
In conclusion, as IgAN progresses, the renal parenchymal VTQ value decreased, indicating the clinical value of VTQ examination in IgAN. The renal parenchymal VTQ value could distinguish IgAN patients from normal people, with high sensitivity and specificity.
![The results of receiver operating characteristic (ROC) curve analysis indicated the diagnostic value of virtual touch quantification (VTQ) values and the combination of renal parenchymal thickness, peak velocity, and VTQ for differentiating patients with immunoglobulin A nephropathy (IgAN) from healthy individuals. A, The VTQ values showed a high diagnostic accuracy in screening IgAN patients from normal people (area under the curve [AUC] = 0.909, sensitivity = 0.828, specificity = 0.867); B, The AUC of combined renal parenchymal thickness, peak velocity, and VTQ values was 0.930, with sensitivity of 0.892 and specificity of 0.867. The results of receiver operating characteristic (ROC) curve analysis indicated the diagnostic value of virtual touch quantification (VTQ) values and the combination of renal parenchymal thickness, peak velocity, and VTQ for differentiating patients with immunoglobulin A nephropathy (IgAN) from healthy individuals. A, The VTQ values showed a high diagnostic accuracy in screening IgAN patients from normal people (area under the curve [AUC] = 0.909, sensitivity = 0.828, specificity = 0.867); B, The AUC of combined renal parenchymal thickness, peak velocity, and VTQ values was 0.930, with sensitivity of 0.892 and specificity of 0.867.](https://services.brieflands.com/cdn/serve/3170b/198f1c9406c0363833e267d16a92ab66011b11e8/iranjradiol-129454-i002-F3-preview.webp)