1. Introduction
Primary breast sarcoma is a rare disease of the breast comprising a heterogeneous group of malignant mesenchymal neoplasms including angiosarcoma, liposarcoma, leiomyosarcoma, fibrosarcoma, sarcomas with bone and cartilage, and malignant fibrous histiocytoma (1). By its very definition, it develops de novo in the breast, not after radiation therapy or in the setting of chronic lymphedema. Adult fibrosarcoma is a malignant neoplasm that is composed of fibroblasts with variable collagen production and classical herringbone architecture, by the world health organization (WHO) classification. Given that there are various malignant mesenchymal neoplasms in the breast; fibrosarcoma is a diagnosis of exclusion.
According to the literature, primary breast sarcoma accounts for less than 1% of all breast cancers in the United States, with an incidence of 44.8 cases per 10 million women per year (2). Due to its rarity, the exact incidence of primary breast fibrosarcoma has not been reported in the literature. Furthermore, there have been few reports (3-7) about radiologic findings of primary fibrosarcoma of the breast in the medical literature.
Here, we present the MRI findings of primary breast fibrosarcoma mimicking invasive ductal carcinoma in a 79-year-old woman who previously underwent interstitial injection mammoplasty.
2. Case Presentation
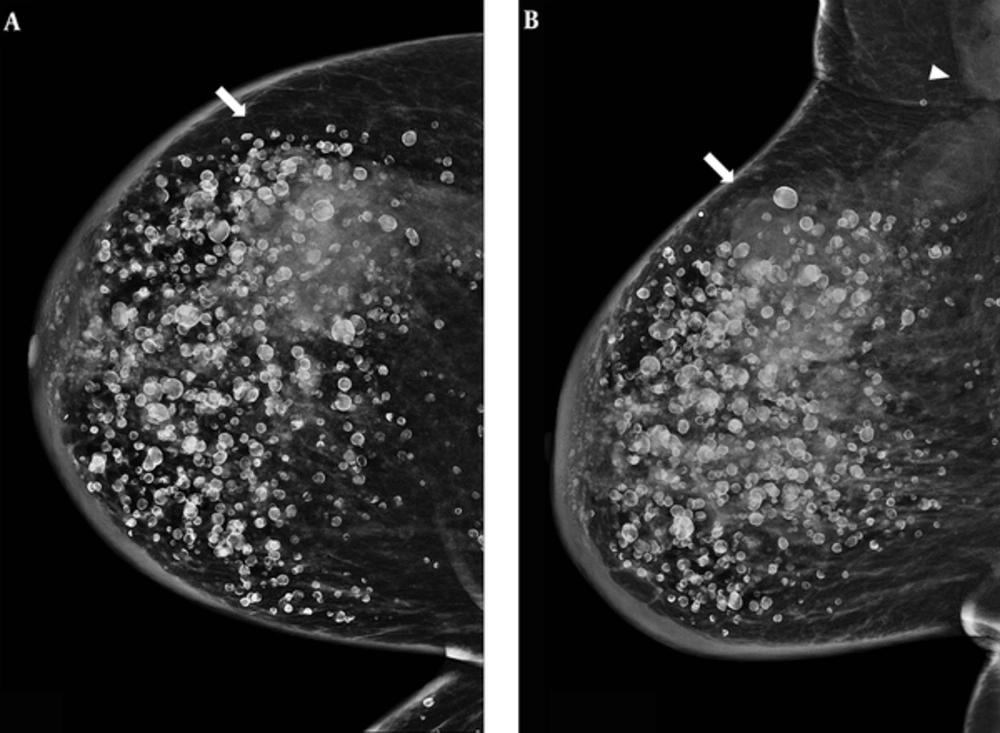
A 79-year-old female patient was referred to our institution for right mastalgia lasting for six months. The patient had a history of free silicone injection for aesthetic breast augmentation 30 years before. Physical examination revealed a palpable mass in the upper outer quadrant of the right breast with inflammation. Mammogram showed numerous diffuse rim calcifications in the bilateral breasts and a huge, irregularly shaped, indistinct margined and hyperdense mass in the upper quadrant of the right breast extending to the right axilla with accompanying lymphadenopathy (Figure 1).
A 79-year-old woman complaining of right mastalgia lasting for six months. This patient had a history of free silicone injection 30 years before. A, Cranio-caudal; B, Mediolateral oblique views of the right breast. A huge irregular shaped, indistinct margined and hyperdense (arrow) mass in the right breast upper quadrant extending to the right axillary lymphadenopathy (arrowhead) with underlying numerous rim calcifications.
Due to artifact associated with the free silicone injection, preoperative MRI was performed instead of breast ultrasound (US) using a 3.0T-MR imaging system (MAGNETOM Skyra, Siemens) with the patient in a prone position. The breast MRI protocol included axial T1- and T2-weighted turbo spin echo images, dynamic sagittal T1-weighted gradient echo images with fat saturation, and axial and coronal three-dimensional (3D) reconstruction images with maximum intensity projection. A T1-weighted scan was performed; TE/TR 717/9.5 ms, matrix size 410 × 512, band width 233 Hz/Px, field of view (FOV) 320 × 320 mm2, slice thickness 3.0 mm, flip angle 120°, voxel size 0.6 × 0.6 × 3.0 mm3. A T2-weighted scan was also performed; TE/TR 3900/110 ms, inversion delay spectral attenuated inversion recovery (SPAIR), matrix size 334 × 512, band width 215 Hz/Px, FOV 340 × 340 mm2, slice thickness 3.0 mm, flip angle 80°, voxel size 0.7 × 0.7 × 3.0 mm3. Pre and dynamic fat-saturated T1-weighted images were obtained with a total of six dynamic acquisitions; TE/TR 4.4/1.7 ms, matrix size 256 × 512, band width 390 Hz/Px, FOV 320 × 320 mm2, slice thickness 3.0 mm, flip angle 10°, voxel size 0.6 × 0.6 × 0.9 mm3. Fifteen milliliters of 0.5 mmol/mL Gadobenate dimeglumine (MultiHance, Bracco Imaging) were injected intravenously followed by a 25 ml saline flush at a rate of 1.5 mL/sec. All collected MRI data were processed using a commercially available computer aided diagnosis (CAD) system (CADstream software, version, 5.2.8.591, Merge Healthcare, Chicago, IL, USA).
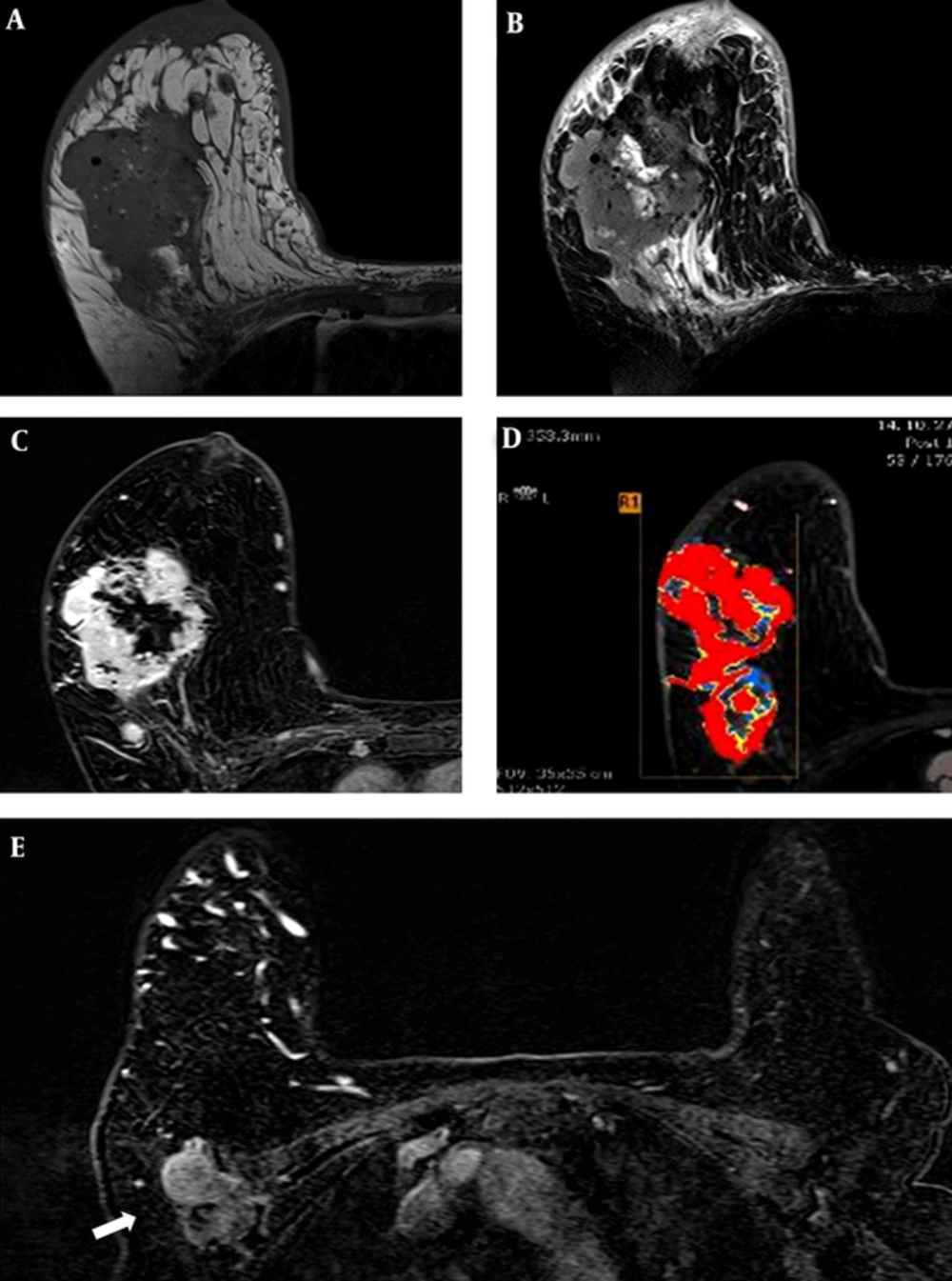
MRI revealed an 11.3 cm × 6.3 cm × 8.5 cm irregularly shaped mass with irregular margins (Figure 2). The mass showed intermediate signal intensity on T1- and T2-weighted imaging (Figure 2A, B). The mass had a centrally necrotic portion that showed low and high signal intensity on T1 and T2 imaging, respectively. The mass demonstrated a heterogeneously enhancing pattern (Figure 2C) with a type III kinetic curve with rapid enhancement and washout (Figure 2D). In the right axilla, there was a 4 cm area of conglomerated lymph nodes suggesting metastasis (Figure 2E). The mass was classified as category 5, highly suggestive of malignancy according to the breast imaging reporting and data system (BI-RADS) and correlated with typical characteristics of invasive ductal carcinoma. Multiple small round nodules in both breasts with high signal intensity on T1 weighted imaging and fat suppression were calcified silicone oil cysts.
MRI of the mass. A, T1-weighted image shows intermediate signal intensity; B, Fat-saturated T2-weighted image also shows intermediate signal intensity with internal high intensity area representing the necrotic portion; C, The mass demonstrates a heterogeneous enhancing pattern with type III kinetic curve; D, Rapid enhancement and washout showing red area in the computer aided diagnosis (CAD) system; E, A 4.4 cm sized metastatic lymph node with internal necrosis in the left axillae is seen on enhanced T1-weighted image (arrow).
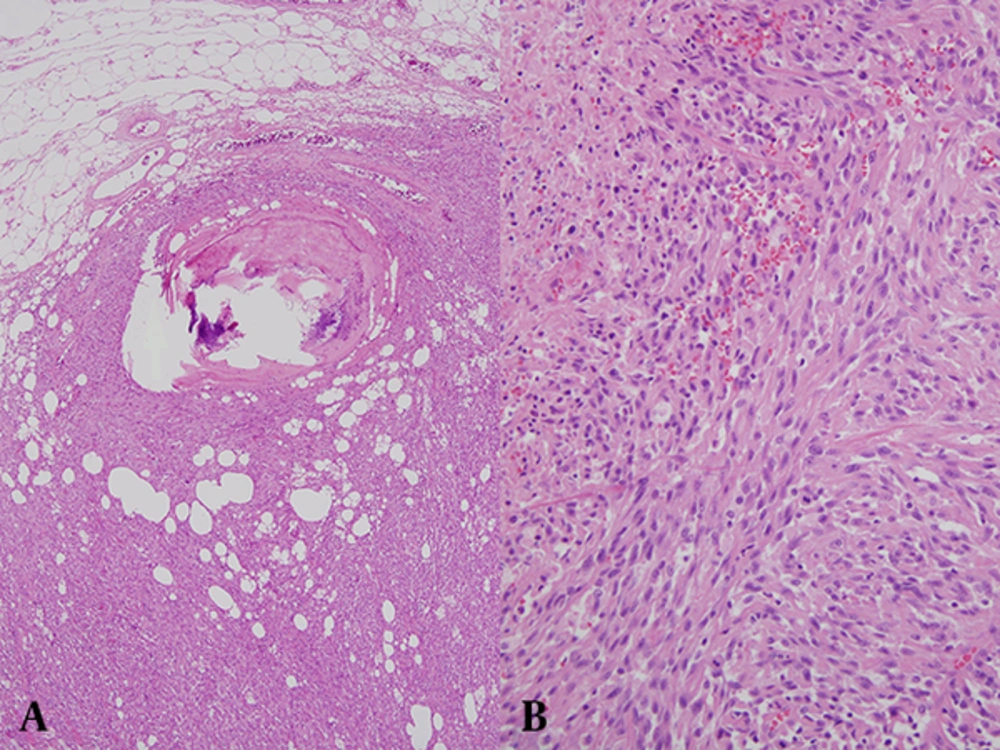
The patient underwent right modified radical mastectomy, which confirmed the presence of fibrosarcoma FNCLCC (French federation of national cancer center) grade 2. On gross examination, an ill-demarcated partially calcified whitish to yellowish mass, measuring 12.5 × 6.7 × 6.3 cm was identified in the right breast. Microscopic examination (Figure 3) revealed the classic herringbone pattern of spindle cells with necrosis (< 50%) and mitosis (14/10 high power field). The pathologic specimen also had multiple oil cysts with rim calcification.
3. Discussion
Our patient’s mass grew rapidly causing her to suffer from severe mastalgia. Because of her prior interstitial silicone injection, it is possible that she overlooked the palpable mass resulting in delayed detection. The initial diagnosis with mammography and sonography was limited because of numerous silicone cyst calcifications, and MRI was required for accurate characterization of the mass. MRI showed intermediate signal intensity on T1 and T2 weighted imaging with heterogeneous enhancement and focal T2 bright high signal intensities, which were considered to represent the necrotic portion of the mass. The kinetic pattern of the lesion was type III with rapid enhancement and washout.
In the fourth edition of the WHO classification of tumors of the breast, there is no classification of fibrosarcoma of the breast. Instead, adult type fibrosarcoma is defined by the fourth edition of the WHO classification of tumors of soft tissue and bone. According to the classification, adult fibrosarcoma is a malignant tumor composed of fibroblasts with variable collagen production and in classical cases, a herringbone architecture (8). There are no known predisposing factors or premalignant lesions. Due to its low incidence, not only the clinical features, but also the radiologic findings of primary breast sarcoma other than breast angiosarcoma have been reviewed as a group, not as an independent etiology.
Breast sarcomas predominantly occur in women in their sixth decade of life (9). The size of primary breast sarcomas varies, ranging from 1 to 30 cm (3). They often present as a palpable, rapidly enlarging and unilateral mass. The mass in our patient had been rapidly increasing in size, consistent with the spectrum of clinical manifestations of breast sarcoma. However, the irregular shape and angular margin in our case was not consistent with previous findings of primary sarcoma, leading to confusion with the finding of invasive ductal carcinoma.
Sarcomas arising in the breast usually do not require elective lymph node dissection because sarcomas tend to spread via hematogenous metastasis rather than nodal metastasis (10). The incidence of nodal metastasis is as low as less 5% according to previous studies (11, 12). From this perspective, axillary lymph node metastasis of primary breast sarcoma is very rare. To our knowledge, there are few case reports of primary breast fibrosarcoma with imaging findings, and the analysis of radiologic findings is limited. Surov et al. (4) reviewed 21 patients with breast sarcoma, and 6 of 21 were histologically diagnosed as fibrosarcoma. In that report, the mammographic findings of primary breast sarcoma presented as nonspecific intramammary masses with architectural distortion. The ultrasound images usually manifested hypoechoic round or oval masses without posterior attenuation. MRI showed breast sarcoma as masses with minimally hyperintense signal intensity on T2 weighted images with inhomogeneous contrast enhancement.
Smith et al. (3) reported 24 cases of breast sarcoma and concluded that the findings of primary breast sarcomas differ from those of breast carcinoma. On mammography masses usually presented as non-calcified oval lesions with indistinct circumscribed margins. On sonography, the lesions appeared as oval solid hypoechoic masses with indistinct margins that exhibited posterior acoustic enhancement and internal hypervascularity. They obtained MRI images in 5 of the 24 cases due to local recurrence, and the lesions showed lobulated and smooth margined and cystic areas on MRI with rapid enhancement and washout pattern of the kinetics curve in two cases.
Santamaria et al. (6) found high T2 signal intensity in breast fibrosarcomas and they insisted that this signal intensity was from the portion where there was a lack of the classic herringbone pattern. Our case showed central high T2 intensity as well, probably representing a necrotic portion.
Primary breast fibrosarcoma is a rare malignancy of the breast characterized by extensive internal necrosis and high T2 signal intensity due to rapid growth, usually without lymph node metastasis. However, we encountered an unusual case of primary breast fibrosarcoma that presented as an irregular and angular margined mass with an appearance typical of invasive ductal carcinoma, including irregularly shaped lymph node enlargement, suggestive of metastases.
In conclusion, radiologists should note that primary breast fibrosarcoma may mimic invasive ductal carcinoma, and when they encounter an unusual mass of the breast, they should consider the possibility of primary breast fibrosarcoma.