Dear Editor,
Neonatal cholestasis (NC) is defined as prolonged elevation of serum levels of conjugated bilirubin beyond two weeks of life. The two most common causes of NC are biliary atresia (BA) and idiopathic neonatal hepatitis (INH). Early and accurate differentiation of these two entities is very important and determines the prognosis and curability of cholestasis. BA requires surgical intervention as soon as possible; whereas, INH needs medical management (1-3). There is no single non-invasive test that can definitely differentiate these two entities. The gold standard for the diagnosis of BA is intraoperative cholangiography. Liver biopsy is the investigation with high diagnostic usefulness in NC. Its diagnostic accuracy for NC has been more than 90% in several studies (1, 4-6). Alagille identified four clinical features that, although nonspecific, supported the correct diagnosis of intrahepatic or extrahepatic cholestasis in 82% of the cases. These clinical variables included stool color within 10 days of admission, age at onset of acholic stools, birth weight, and the features of hepatic involvement, specifically the presence of hepatomegaly and consistency of the liver in palpation (6).
We conducted a retrospective study between March 1996 and March 2007 to review medical records of infants with NC. Patients were all admitted in Children’s Hospital Medical Center affiliated to Tehran University of Medical Sciences, which is the major tertiary pediatric referral center in Iran. We reviewed medical records of 250 infants with NC. Infants were excluded if they did not have liver biopsy results, definite diagnosis or complete data on their medical records. Ultimately, 88 infants with BA confirmed by exploratory laparotomy or INH confirmed by liver biopsy were enrolled into the study. In this study, the gold standard tests were liver biopsy and exploratory laparotomy in INH and BA cases, respectively. The sensitivity, specificity and predictive values of HIDA scan and Alagille criteria were evaluated in comparison with liver biopsy and surgical findings. Chi-square test was used for comparison and P < 0.05 was taken as significant.
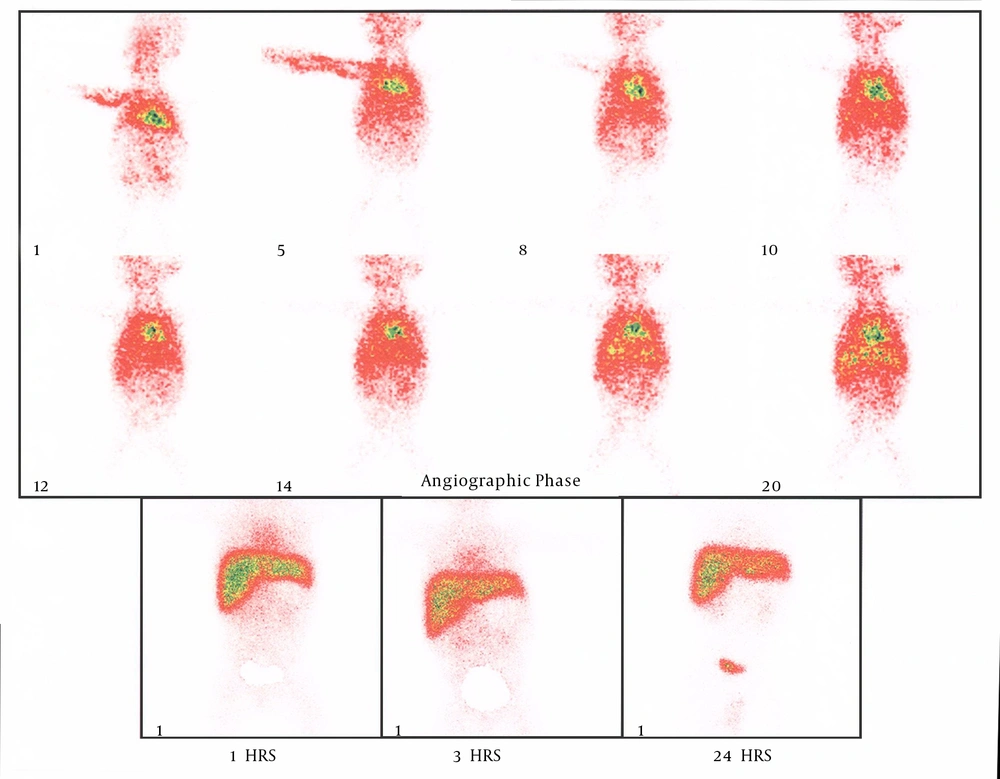
There were 88 infants with NC, including 49 (55.7%) boys and 39 (44.3%) girls. Of these, 63 (37 boys, 26 girls) were diagnosed to have INH and the remaining 25 (12 boys, 13 girls) had BA, according to liver biopsy (P = 0.361). Laparotomy confirmed liver biopsy results in all 25 cases of BA. It means that liver biopsy had 100% accuracy in diagnosing BA. Thirty-three patients had HIDA scan. Among the 23 cases with a positive scan for BA (Figure 1), 14 of them also had BA in liver biopsy (P = 0.031). Among 15 patients with a positive liver biopsy for INH, HIDA scan had shown INH in only two cases (P = 0.15).
Among 18 cases who had all four criteria of Alagille accommodated with INH, 14 (77.8%) cases had INH in liver biopsy (P = 0.0514). Of the five patients who had all four Alagille criteria accommodated with BA, in two (40%) cases, liver biopsy showed BA (P = 0.554). So the accuracy of Alagille criteria in our study is lower than what is reported in the pediatric GI textbooks. HIDA scan had a diagnostic accuracy of 60% and 11% for BA and INH, respectively.
In conclusion, there is not a single imaging test or clinical feature that has sufficient sensitivity and specificity to differentiate BA from INH, but according to our study hepatobiliary scintigraphy had a good sensitivity for diagnosing BA and the presence of acholic stools and hepatomegaly is significantly higher in infants with BA. So, scintigraphy and clinical features can help in the assessment of any infant with NC to arrive at a timely diagnosis for appropriate management. Since percutaneous liver biopsy is highly accurate in diagnosing BA, in developing countries such our country, using this modality is cost-effective, and it can preclude unnecessary surgical exploration.