1. Background
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is a rare autosomal dominant genetic vascular dysplasia with an estimated prevalence of 1 to 2 cases per 10,000 (1, 2). Multiple organs can be involved in HHT; however, the clinical symptoms may have no specificity, which generally delays the diagnosis and treatment of HHT. Liver involvement is more common with a previous incidence of 8 - 31%, which has been increased to about 74 - 79% (3-5). Liver involvement in patients with HHT has not been fully characterized. The rapidly developing imaging technology, in particularly the multidetector computed tomography (MDCT), effectively facilitates the diagnosis of HHT. Although there is some previous literature discussing liver involvement of HHT, it is still rare to see a comprehensive discussion that also addresses both intrahepatic shunts and biliary diseases, especially the dynamic alteration of biloma via high quality CT images.
2. Objectives
The purpose of the present study is to characterize the liver involvement of HHT via CT images in conjunction with intrahepatic shunts.
3. Patients and Methods
3.1. Clinical Data
Between January of 2009 and June of 2014, a group of 12 patients with liver involvement of HHT in our hospital underwent dynamic contrast enhanced CT, and the scans were reviewed retrospectively. These 12 patients (including 11 women and 1 man), were aged between 42 - 68 years, with a mean of 54.8 years. The clinical manifestations included repeated epistaxis, right upper quadrant abdominal pain, liver dysfunction, abdominal distension, fatigue, and loss of appetite.
3.2. Confirmed Diagnosis of HHT
The patients with chronic liver disease and tumor-like lesions, such as hepatitis B, hepatitis C, autoimmune liver disease, alcoholic liver disease, drug-induced liver disease, hemangioma, and primary liver cancer were all excluded from our study. The diagnosis of HHT requires the presence of at least three of the following criteria (6): (i) recurrent spontaneous epistaxis; (ii) mucocutaneous telangiectasia; (iii) a family medical history; and (iv) the presence of visceral involvement. All 12 patients were diagnosed with HHT since they all met at least three of the criteria. The clinical data and medical history of the 12 patients are shown in Table 1. In this study, the visceral involvement included the liver, lung, pancreas, spleen, and small intestine were detected by MDCT.
| No. | Gender | Age | Epistaxes | Telangiectasia | Family History | Visceral Involvement |
|---|---|---|---|---|---|---|
| 1 | F | 55 | + | + | + | Liver |
| 2 | F | 60 | + | + | - | Liver |
| 3 | F | 60 | + | - | + | Liver, Pancreas |
| 4 | F | 45 | + | - | + | Liver |
| 5 | F | 46 | + | + | + | Liver, Pancreas, Small Intestine |
| 6 | F | 54 | + | + | + | Liver, Lung, Pancreas |
| 7 | F | 59 | + | - | + | Liver, Pancreas |
| 8 | F | 56 | + | + | - | Liver, Lung |
| 9 | M | 42 | + | + | - | Liver, Spleen |
| 10 | F | 52 | + | + | + | Liver, Lung |
| 11 | F | 68 | + | + | + | Liver |
| 12 | F | 54 | + | + | + | Liver, Pancreas |
Abbreviation: F, female, M, male.
3.3. Scanning
Dynamic contrast enhanced CT was performed on all of the patients. Sixty four-section spiral CT (GE Light-Speed VCT, Milwaukee, WI, USA) was performed on the region of clinical interest with patients in the supine position with the following parameters: section thickness of 5 mm, intervals of 5 mm, rotation time of 0.5 seconds, 120 kV, and 380 mAs. Section thickness and spacing of reconstruction were 0.625 mm. An intravenous injection of nonionic contrast medium (iodine amine, 370 mg I/mL, 100ml) via the cubital vein was provided for the contrast enhanced scanning at a flow rate of 3 ml/s. The scanning of the arterial phase, the venous phase, and the delayed phase were approximately 20s ~ 25 s, 60s ~ 70 s, and 180 s, respectively. Maximal intensity projection (MIP) images directly from the CT console for the three phases were reconstructed using a 0.625 mm interval, and the image data were sent to the workstation (GE ADW 4.3) for review and analysis. Two radiologists with 10 and 18 years of experience in abdominal and vascular imaging, respectively, reviewed the original images and reconstructions at the workstation. They were blind to the clinical data, and a consensual interpretation was reached by discussion. The radiologists also evaluated the presence and type of shunts.
4. Results
4.1. Visceral Involvements
Among all of the patients, 4 had cases of single liver involvement, and 8 had cases accompanied by other organ involvement including the pancreas (5 cases), lung involvement (3 cases), the spleen (1 case), and the small intestine (1 case).
4.2. Scanning Findings
Liver enlargement was observed by MDCT scanning, and intrahepatic anomalies during the contrast enhanced scanning could be detected (Table 2).
| No. | Parenchymal Telangiectasias | Intrahepatic Shunts | Large Confluent Vascular Masses | Biliary Diseases | Abnormal Origin of Hepatic Artery |
|---|---|---|---|---|---|
| 1 | + | A-V | - | Biloma | RHA from SMA |
| 2 | + | A-V | - | Slight ectasia | - |
| 3 | + | A-V | - | Biloma | ALHA from LGA |
| 4 | + | A-V | - | Slight ectasia | - |
| 5 | + | A-V | - | Slight ectasia | LHA from LGA |
| 6 | + | A-V | - | Biloma | - |
| 7 | + | A-P | + | - | RHA from SMA |
| 8 | + | A-P | - | - | - |
| 9 | + | P-V | + | Slight ectasia | - |
| 10 | + | P-V | - | Slight ectasia | - |
| 11 | + | P-V | - | - | - |
| 12 | + | P-V | - | - | - |
Abbreviations: ALHA, accessory left hepatic artery; A-P, arterioportal shunt; A-V, arteriovenous shunt; LGA, left gastric artery; LHA, left hepatic artery; P-V, portovenous shunt; RHA, right hepatic artery; SMA, superior mesenteric artery.
4.2.1. Hepatic Vascular Anomaly
4.2.1.1. Intrahepatic Shunts
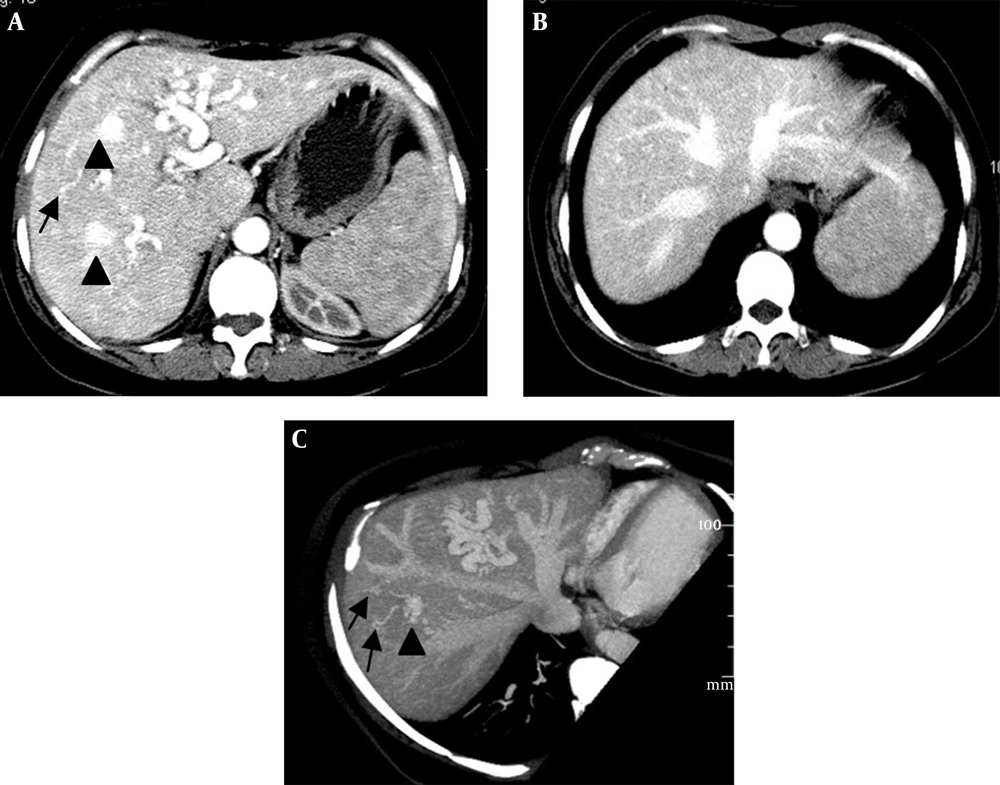
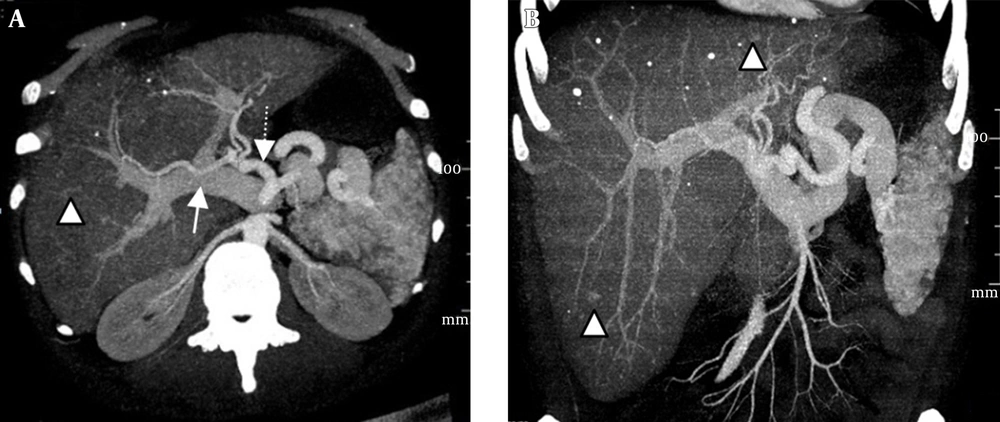
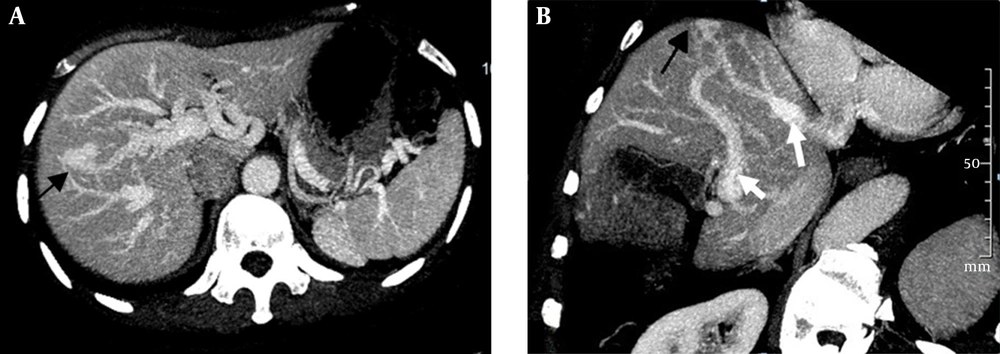
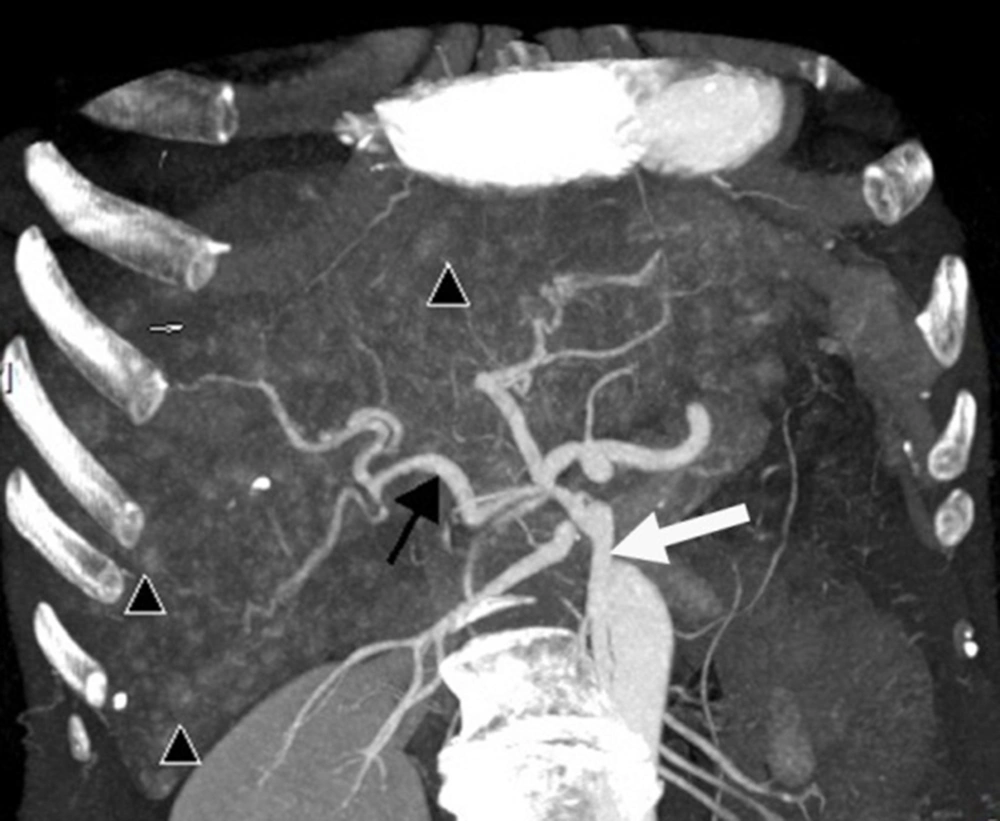
(i) An arteriovenous shunt was present in 6 cases (50%). Dilation and tortuosity of the hepatic arteries and early opacification of the main hepatic veins could be observed in the arterial phase (Figure 1A and 1B). The diameters of the hepatic arteries ranged from 8 mm ~ 11 mm. Some large confluent vascular masses and several small telangiectasia were also detected (Figure 1A and 1C). (ii) An arterioportal shunt was found in 2 cases. Early opacification of the portal vein was detected during the arterial phases in the contrast-enhanced CT scans (Figure 2A), and tubular or nodular capillaries were observed around the hepatic periphery (Figure 2B). (iii) A portal venous shunt was detected in 4 cases. A dilated portal vein and a hepatic vein were revealed during the venous phase, and a focal vascular mass was found between the portal vein and hepatic vein (Figure 3A). Oblique MIP images more precisely showed the lesions (Figure 3B).
Intrahepatic arteriovenous shunts. A and B, Contrast-enhanced CT shows a dilated hepatic artery and early opacification of the main hepatic veins during the arterial phase; C, Oblique maximal intensity projection MDCT in the parasagittal plane shows a dilated hepatic artery and early simultaneous opacification of the hepatic veins; A and C, Large confluent vascular masses (black arrowheads) and multiple telangiectasias (black arrows) in the peripheral parenchyma are visible.
Intrahepatic arterioportal shunts. A and B, Reconstructed images of maximal intensity projection in the parasagittal and coronal plane show early opacification of the portal veins (white arrow) and arteries (dashed arrow). The presence of multiple telangiectasias (white arrowheads) is evident. (Note: These tiny dense highlighted foci in both images, near the described telangiectasias and even outside of the hepatic field, are calcifications.)
Intrahepatic portal venous shunts. A, Axial; B, Oblique maximal intensity projection images obtained in the venous phase show portal venous shunting, with the dilated portal vein (short white arrow) communicating with the hepatic vein (long white arrow) through a focal vascular mass (black arrow).
4.2.1.2. Telangiectasia
Telangiectasia was detected during the arterial phases in 8 patients. The vascular ectasias appeared to be round, with a diameter of 5 - 7 mm and a prevalently peripheral arrangement (Figure 1C and 2B). Diffuse and focal distributions were present in 6 cases and 2 cases, respectively.
4.2.1.3. Large Confluent Vascular Masses
Large confluent vascular masses were detected during the arterial phases, with diameters ranging from 5 to 20 mm (Figure 1C and 3A).
4.2.2. Biliary Disease
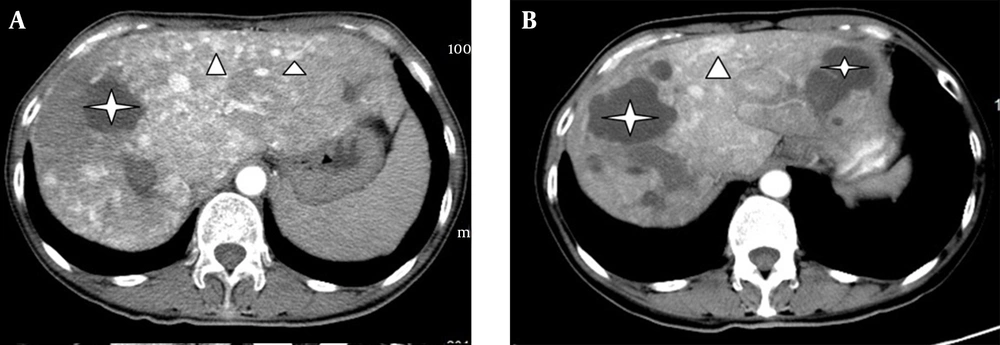
Biliary diseases were found in 8 patients, with 3 cases showing multiple round or tubular low density lesions with ill-defined boundaries and no enhancement in liver parenchyma in the MDCT images (Figure 4A). One of the 3 patients, who was suspected of having a liver abscess, received drainage treatment. No significant changes were shown after routine treatment, and the drained fluid was confirmed to be bile without bacteria. However, in another patient, an intrahepatic cyst grew in size after 8 months without any treatment (Figure 4B). These 3 patients showed elevated alkaline phosphatase levels (ALP, mean value of 271 per unit, ranging from 151 to 499 per unit), normal total bilirubin (TBil), and an accompanying intrahepatic arteriovenous shunt.
The remaining 5 patients had slight bile duct ectasia near the porta hepatis without abnormal ALP and TBil levels. Three of them had arterioportal shunts and 2 had portal venous shunts.
4.2.3. Vascular Anatomic Variants
Anatomic variants of the hepatic arterial supply were detected in 4 patients. A right hepatic artery originating from the superior mesenteric artery was evident in 2 patients (Figure 5), and a left hepatic artery and an accessory left hepatic artery were observed as originating from the left gastric artery in 2 patients.
4.2.4. Liver Biopsy Pathology
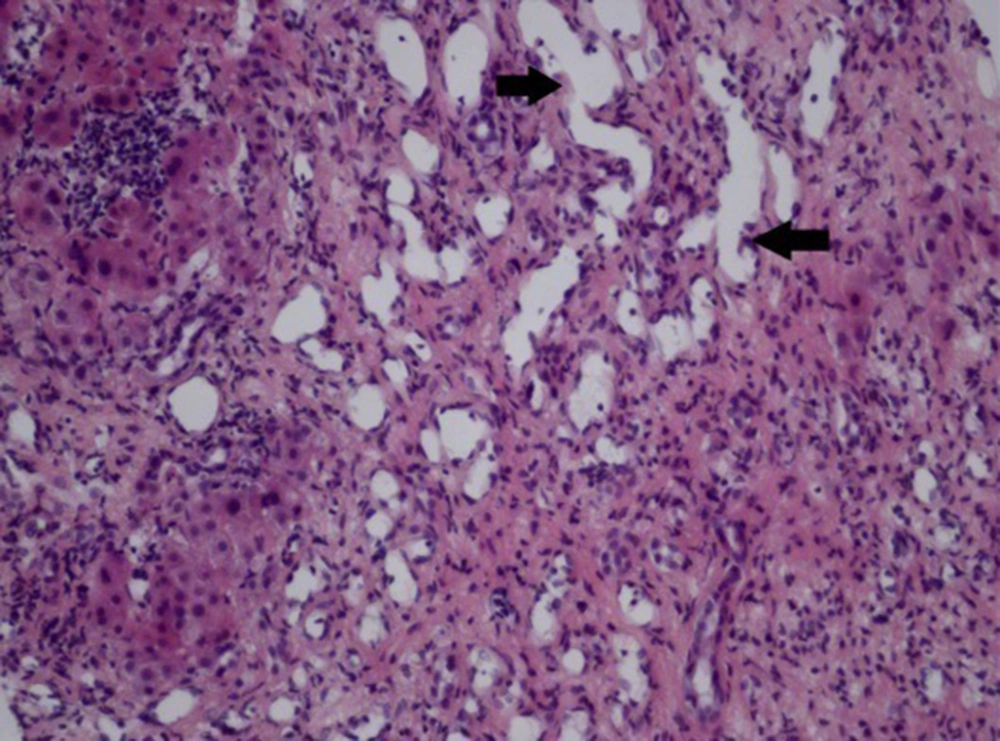
Only 1 patient underwent liver biopsy, which was characterized by fibrous tissue and capillary hyperplasia, hepatic cord atrophy, hyperplastic vascular ectasia, and angiomatous disorders (H & E 200×) (Figure 6).
5. Discussion
HHT, or Osler-Weber-Rendu disease, is an autosomal dominant disorder characterized by angiodysplastic lesions (telangiectasias and arteriovenous malformations) that may involve many organs (7). Repeated intractable epistaxis is the main clinical manifestation of HHT. It has been reported that about 93% patients with HHT sustain epistaxis that is ingravescent with age (8). Sometimes, hemoptysis, melena, hematuresis, fundus hemorrhage, and hypermenorrhea can also be observed. Telangiectasias are observed in about 25% patients with HHT, with partially revealed multi-organ involvement, such as in the chest, abdomen, and encephalic region.
The incidence of HHT has gradually been increasing in recent years due to the rapid evolution of MDCT technology. MDCT is capable of clearly displaying vascular lesions and hemorrhage within substantive viscera, especially the corresponding post-processing technology, such as MIP. Therefore, it can facilitate in diagnosing the hepatic vascular abnormalities and the various types of shunting (9). The reasons underlying epistaxis had remained unknown for all patients in our study before MDCT, until review of their medical histories was performed in order to determine the diagnosis of HHT.
Liver is considered to be the most commonly involved organ in HHT (4). The prevalence of hepatic involvement in HHT was estimated to be between 8 - 31% by retrospective studies with single-detector CT (4). However, more recent studies with multidetector CT for consecutive patients have demonstrated that its prevalence is much higher, ranging from about 74 - 79% (3, 5). Nearly half or even a majority of the patients with HHT were asymptomatic (10). Ianora et al. (3) assessed the liver condition of 70 patients with HHT using multiphasic CT and found liver abnormalities in 52 patients in CT scans. However, only 4 of the 52 patients were symptomatic. In our group, most of the patients experienced clinical symptoms of abdominal distension, right upper quadrant abdominal pain, and fatigue, with no specificity. Additionally, the single-involvement of the liver was rare in the HHT patients, and other visceral involvements were concurrent with an incidence rate of approximately 66.7% in our group.
In our study, one major discovery via liver scanning was the detection of intrahepatic shunting, which can be divided into 3 types: 1) Arteriovenous shunt is the most common. A large quantity of arteriovenous shunts may induce congestive heart failure, leading to hepatomegaly, pulmonary hypertension, and heart failure. Long-term liver ischemia and hypoxia may cause liver fibrosis and hepatic cirrhosis. The incidence of arteriovenous shunts in our group was about 50%, and these patients typically suffered from fatigue and cyanosis with no hepatic cirrhosis after mild physical activity. CT scanning revealed liver hypertrophy and contrast enhanced CT indicated dilation and tortuosity of the hepatic arteries, as well as early opacification of the main hepatic veins during the arterial phase; 2) Arterioportal shunts rarely occur according to the literature, and when they do, they are always accompanied by arteriovenous shunts. It has been demonstrated that they are related to portal hypertension and corresponding complications, such as hemorrhage of the digestive tract or abdominal dropsy. Contrast enhanced CT scans revealed early opacification of the portal vein during the arterial phase in 2 cases in our study; 3) Portovenous shunt has been rarely reported because it can hardly be detected by routine scanning or angiography, except by microscopy (10, 11). However, it has been reported that in an advanced stage of involvement, large portal venous shunts are more likely to be present than the other types of shunts, and the patients with portal venous shunts are older than those without portal venous shunts (12). In our study, dilated portal veins and hepatic veins were revealed during the venous phase in 4 cases, and tortuous ectatic vasculars were clearly observed between the portal vein and the hepatic vein in MIP images. We therefore speculated that MIP might be advantageous in revealing intrahepatic vascular lesions. According to these radiological findings, the possibility of the development of arteriovenous shunts and arterioportal shunts has been ruled out. Due to the elderly age of these patients (all aged 42-68 years), age is less likely to be related to the shunt types. In addition, it was revealed that MDCT and the reconstruction programs such as MIP are more favorable in identifying and characterizing the complex vascular alterations typical of HHT (13).
Apart from the shunting, telangiectasias and large confluent vascular masses are also important early findings of the intrahepatic vascular variations, with incidence rates of about 66.7% and 13.3%, respectively (9). Moreover, abnormal parenchymal perfusion was reported to occur in the later period of HHT with arterioportal shunts. Such a finding was not detected in our study, which was speculated to be related to the course of HHT.
The incidence of the subtype of intrahepatic biliary disease with the liver involved in HHT was high (14), with the main manifestations of biliary stenosis and local cystic dilation (14, 15). In our group, 8 out of 12 cases were detected with biliary diseases. The reasons underlying bile duct abnormalities may be the pressure caused by tortuous ectatic vasculars (16, 17). For instances, the 5 cases in our group had slight bile duct ectasy near the porta hepatis, and these patients were all asymptomatic with no elevated ALP. However, serious biliary abnormalities occurred due to biliary ischemia, which was induced by intrahepatic shunting. For instances, the 3 cases in our group had circular or tubular bile cysts. In the literature, it has been reported that hepatic artery stenosis after liver transplantation or blockage may also lead to biliary ischemia and necrosis (18), which is in agreement with our findings. Arteriovenous shunts were found in the 3 patients with biliary diseases, indicating that different types of shunting might lead to different degrees of biliary ischemia, and arteriovenous shunting might induce the most serious ischemia, which increases the risk of serious biliary diseases.
In most of the previous reports, these biliary diseases have been described as biliary cysts. However, we believe that the biloma may be a better nomination for the cystic lesions due to the absence of clinical symptoms of cholangiectasis and jaundice. The obvious growth of the biloma after 8 months without treatment in one patient suggested that the biliary diseases progress along with aggravating biliary ischemia. A study reported by Wu et al. proved that biliary diseases occur in the late period of HHT, and its occurrence is closely associated with liver ischemia (17).
Vascular anatomic variants were detected in about 33.3% of patients (4 cases) in our group, which is higher than that reported in previous literature. Three of the 4 cases were detected with accompanying arteriovenous shunts, and the other case with an accompanying portal venous shunt. However, it is unlikely that the relationship between the shunt types and HHT can be precisely defined (17). Still, the data about the anatomic variants of hepatic vasculature is of significance in preoperative assessment, such as in determining candidates for liver transplantation.
Liver biopsy was performed in only one patient in our study, and abnormal ectatic vessels were observed in the specimens of the biopsy. Although it facilitates the diagnosis of intrahepatic vascular disorders, the diagnosis of HHT is best defined based on both clinical and radiological examinations. Given the negative findings by histological examination of the liver in establishing the presence of liver involvement or in classifying the type of liver disease in patients with HHT, this potentially risky procedure is unnecessary (18).
In conclusion, liver involvement by HHT is primarily characterized by diffuse liver vascular shunting and biliary diseases. Specifically, arteriovenous shunts are more likely to induce biloma, and biloma may progress along with aggravating biliary ischemia.