1. Background
First introduced in 1982, totally implanted venous access ports (TIVAPs) were extensively used in oncologic patients. These devices have been used as a safe method for avoiding venous injury and improving the quality of life for cancer patients (1-5).
Major complications after placement of the TIVAP include infection, thrombosis, catheter migration, occlusion, and central vein stenosis (6). Among these, venous thrombosis is the second major complication associated with TIVAPs after infection (5). Venous thrombosis caused by TIVAPs has been associated with high morbidity and additional costs (5). The manifestations of thrombosis can range from no clinical importance to serious complications (7). Vessel injury caused by catheter insertion, venous stasis caused by an indwelling catheter, and cancer-related hypercoagulability all contribute to the development of venous thrombosis (8).
The incidence of venous thrombosis based on access route after implantation of the TIVAP is controversial. Biffi et al. (9) reported that the rate of venous thrombosis was not significantly different between subclavian and internal jugular vein access (9). However, Araujo et al. (10) reported lower long-term morbidity rates including venous thrombosis with internal jugular vein rather than subclavian access. Symptomatic TIVAP-related venous thrombosis remains relatively rare (5). However, to the best of our knowledge, characteristics of thrombosis after TIVAP implantation via access of the axillary vein has not been reported.
2. Objectives
In the present study, the incidence and characteristics of venous thrombosis associated with TIVAP via the axillary vein in cancer patients were evaluated.
3. Patients and Methods
This retrospective cohort study was approved by the Institutional Review Board of our hospital, and the need for informed consent was waived. A total of 4,773 consecutive cancer patients (male to female ratio [M:F] = 2,508:2,265; mean age ± SD: 60.6 ± 12.0 years) who received TIVAPs via the axillary vein between May 2012 and July 2018 were retrospectively reviewed. Symptomatic axillary vein thrombosis was defined as pain, swelling, and color change of the ipsilateral upper extremity with thrombosis of the axillary vein at the implantation site. Among the 4,773 patients, 18 patients experienced symptomatic axillary vein thrombosis associated with a TIVAP during the study period. Baseline demographic characteristics of patients are shown in Table 1.
| Number of TIVAP implantations (N = 4,773) | Number of venous thromboses (N = 18) | |
|---|---|---|
| Age, y | 60.6 ± 12.0 | 66.2 ± 7.5 |
| Gender | ||
| Male | 2508 (52.6) | 14 (77.8) |
| Female | 2265 (47.4) | 4 (22.2) |
| Type of cancer | ||
| Breast cancer | 1049 (21.9) | 1 (5.6) |
| Lung cancer | 882 (18.4) | 7 (38.8) |
| Stomach cancer | 510 (10.6) | 0 |
| Colon cancer | 419 (8.7) | 0 |
| Pancreatic cancer | 252 (5.2) | 4 (22.2) |
| Lymphoma | 250 (5.2) | 0 |
| Rectal cancer | 214 (4.4) | 1 (5.6) |
| Esophageal cancer | 128 (2.6) | 1 (5.6) |
| Ovarian cancer | 111 (2.3) | 0 |
| Hypopharyngeal cancer | 35 (0.7) | 1 (5.6) |
| Klatskin tumor | 16 (0.3) | 1 (5.6) |
| Parotid gland cancer | 8 (0.1) | 1 (5.6) |
| Others | 899 (19.6) | 1 (5.6) |
| TIVAP insertion side | ||
| Right | 3769 (78.9) | 15 (83.3) |
| Left | 1004 (21.1) | 3 (16.7) |
Abbreviation: TIVAP, totally implanted venous access port
aValues are expressed as mean ± SD or No. (%).
All TIVAPs were inserted using the single incision technique reported by Seo et al. (11) via the axillary vein. Before the procedure, the state of the brachiocephalic vein and superior vena cava was evaluated on a chest computed tomography (CT) scan if available. In all patients, the axillary vein was punctured under ultrasound guidance using a micropuncture needle. The intended location of the catheter tip was between the upper third and center of the right atrium on fluoroscopic image in supine position. After confirmation of catheter function by regurgitation of blood with a syringe, the final length of the catheter was determined. TIVAPs were inserted under fluoroscopic guidance by attending interventional radiologists. Celsite Discreet (B.Braun Medical, Boulogne Cedex, France) STR and STL TIVAPs equipped with a 6.5F silicone catheter were used for access via the left (n = 1,004) and right (n = 3,769) veins, respectively. Initially, single incision technique was performed via the left axillary vein. After February 2014, single incision technique was performed via the right axillary vein due to the risk of stenosis of the left innominate vein. Several indications were also present in some cases, such as the contralateral side of breast cancer, contralateral side of lung cancer, and contralateral side of small or deep location of the axillary vein. Radiologic findings for venous thrombosis were evaluated based on CT including scans of the axillary vein. Among the 18 patients with symptomatic axillary vein thrombosis, thrombosis was diagnosed on the upper extremity (UE)-CT venography in 15 patients and on chest CT in three patients. Presence of edema in both upper extremities, presence of thrombus, resolution of thrombus, and catheter tip location were evaluated on initial CT images and follow-up CT images. Medical records were retrospectively reviewed to evaluate cancer types, symptoms, onset time of symptoms after TIVAP insertion, body mass index (BMI), smoking status, presence of metastasis, history of thrombosis, and Khorana score for risk of thrombosis.
Statistical analysis was performed using SPSS® (version 20.0; SPSS Inc., Chicago, IL, USA). Demographic data and clinical features were analyzed using descriptive methods. Patient baseline characteristics were summarized using mean and range for continuous variables and frequency and percentages for categorical variables. Age, gender, type of cancer, and insertion side of TIVAP were analyzed. To determine risk factors for symptomatic thromboses, univariable analyses (using the chi-square test, Fisher`s exact test, and the Student’s t-test) and multiple logistic regression analysis were used. P values < 0.05 were considered statistically significant.
4. Results
Demographic characteristics of the patients and results are shown in Tables 1 and 2. The incidence of symptomatic axillary vein thrombosis was 0.38% (18/4,773). The 18 patients with symptomatic axillary vein thrombosis included 14 males and four females, and the mean age was 66.2 years (range: 53 - 82 years). Based on univariable analyses, age, gender, and type of cancer were significantly associated with the risk of symptomatic axillary vein thrombosis (Table 3). In the18 patients, the cancer types included lung cancer (n = 7), pancreatic cancer (n = 4), and other tumors (breast cancer, esophageal cancer, hypopharyngeal cancer, Klatskin tumor, parotid gland cancer, rectal cancer, and tonsillar cancer). The incidence rates for symptomatic axillary vein thrombosis based on cancer types were 0.79% (lung cancer, 7/882) and 1.58% (pancreatic cancer, 4/253). Multivariable binary logistic regression analysis revealed that pancreatic cancer (P = 0.012, odds ratio: 16.903) and lung cancer (P = 0.047, odds ratio: 8.384) were significantly associated with the risk of symptomatic axillary vein thrombosis (Table 4). Age and gender were not significantly associated with the risk of symptomatic axillary vein thrombosis on multivariable binary logistic regression analysis. Regarding implantation site, symptomatic axillary vein thrombosis developed in patients on the right side (15/3,769, 0.39%) and on the left side (3/1,004, 0.29%). Implantation site was not significantly associated with the risk of symptomatic axillary vein thrombosis (Table 3).
| Number | Age/sex | Underlying malignancy | Onset timea | Insertion side | Tip locationb | UE edema | Resolution of thrombus | UE symptom | BMI | Smoking status | Metastasis | Khorana model |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68/M | Tonsillar cancer | 195 | Left | 2 | Yes | Yes | Pain, swelling | 20.2 | EX-Smoker | Yes | Intermediate, 1 |
| 2 | 72/M | Rectal cancer | 44 | Right | 2 | Yes | No | Pain, swelling, color change | 23 | Smoker | No | Low, 0 |
| 3 | 69/M | Parotid gland cancer | 287 | Right | 2 | Yes | Yes | Swelling | 21 | EX-Smoker | Yes | Low, 0 |
| 4 | 72/F | Pancreatic cancer | 6 | Right | 2 | Yes | No | Pain, swelling | 24 | Non-smoker | Yes | High, 3 |
| 5 | 69/M | Pancreatic cancer | 6 | Right | 2 | Yes | Yes | Swelling | 22 | Ex-smoker | Yes | High, 3 |
| 6 | 61/M | Pancreatic cancer | 64 | Left | 2 | No | Yes | Swelling | 23 | Non-smoker | Yes | High, 5 |
| 7 | 75/M | Pancreatic cancer | 154 | Right | 2 | Yes | Yes | Swelling | 26 | Non-smoker | No | Intermediate, 2 |
| 8 | 82/M | Lung cancer | 292 | Right | 2 | Yes | No | Swelling, color change | 23 | Ex-smoker | Yes | High, 3 |
| 9 | 57/M | Lung cancer | 27 | Right | 2 | Yes | Yes | Swelling | 22 | Ex-smoker | Yes | High, 3 |
| 10 | 53/F | Lung cancer | 7 | Right | 1 | No | No | Swelling | 28 | Non-smoker | Yes | Intermediate, 2 |
| 11 | 57/M | Lung cancer | 124 | Right | 2 | N/A | Yes | Swelling | 24 | Non-smoker | No | Intermediate, 2 |
| 12 | 73/F | Lung cancer | 13 | Right | 2 | Yes | No | Swelling | 21 | Non-smoker | Yes | Intermediate, 2 |
| 13 | 65/M | Lung cancer | 21 | Right | 2 | Yes | No | Swelling | 20 | Ex-smoker | Yes | Intermediate, 2 |
| 14 | 68/M | Lung cancer | 20 | Right | 2 | Yes | Yes | Swelling | 23 | Ex-smoker | No | Intermediate, 2 |
| 15 | 59/M | Klatskin tumor | 27 | Right | 2 | Yes | Yes | Swelling | 23 | Non-smoker | No | Low, 0 |
| 16 | 64/M | Hypopharyngeal cancer | 154 | Right | 1 | N/A | Yes | Swelling | 25 | Ex-smoker | No | Low, 0 |
| 17 | 59/M | Esophageal cancer | 9 | Right | 2 | Yes | Yes | Swelling | 22 | Ex-smoker | No | Low, 0 |
| 18 | 70/F | Breast cancer | 85 | Left | 2 | N/A | Yes | Swelling | 26 | Non-smoker | No | Low, 0 |
Abbreviations: BMI, body mass index; UE, upper extremity.
aOnset time after TIVAP insertion (day).
bTip location (1, superior vena cava; 2, from the distal third of the superior vena cava to right atrium).
| Non-thrombosis group (N = 4,755) | Thrombosis group (N = 18) | P value | |
|---|---|---|---|
| Age, y | 60.6 ± 12.0 | 66.2 ± 7.5 | 0.005b |
| Gender (male) | 2494 (52.5) | 14 (77.8) | 0.032c |
| Type of cancer | 0.008d | ||
| Breast cancer | 1048 (22.0) | 1 (5.6) | |
| Lung cancer | 875 (18.4) | 7 (38.8) | |
| Stomach cancer | 510 (10.7) | 0 | |
| Colon cancer | 419 (8.8) | 0 | |
| Pancreatic cancer | 248 (5.2) | 4 (22.2) | |
| Lymphoma | 250 (5.2) | 0 | |
| Rectal cancer | 213 (4.5) | 1 (5.6) | |
| Esophageal cancer | 127 (2.7) | 1 (5.6) | |
| Others | 1065 (22.5) | 4 (22.2) | |
| TIVAP insertion side | 1.00 | ||
| Right | 3754 (78.9) | 15 (83.3) | |
| Left | 1001 (21.1) | 3 (16.7) |
Abbreviation: TIVAP, totally implanted venous access port
aValues are expressed as mean ± SD or No. (%).
bStudent’s t-test.
cChi-square.
dFisher’s exact test.
| OR | 95% CI | P value | |
|---|---|---|---|
| Age, y | 2.085 | 0.590 - 7.376 | 0.254 |
| Gender | 0.236 | ||
| Male | 1 | ||
| Female | 1.030 | 0.981 - 1.082 | |
| Type of cancer | |||
| Breast cancer | 1 | 0.383 | |
| Lung cancer | 8.384 | 1.030 - 68.274 | 0.047 |
| Stomach cancer | 0 | 0 | 0.994 |
| Colon cancer | 0 | 0 | 0.994 |
| Pancreatic cancer | 16.903 | 1.881 - 151.893 | 0.012 |
| Lymphoma | 0 | 0 | 0.996 |
| Rectal cancer | 4.920 | 0.307 - 78.970 | 0.261 |
| Esophageal cancer | 8.252 | 0.513 - 132.737 | 0.136 |
| Others | 3.936 | 0.439 - 35.275 | 0.221 |
| TIVAP insertion side | 0.799 | ||
| Right | 1 | ||
| Left | 1.181 | 0.327 - 4.274 |
Abbreviations: CI, confidence interval; OR, odds ratio; TIVAP, totally implanted venous access port
aRegressions include adjustments for age, gender, type of cancer, and TIVAP insertion side.
The median time between insertion of a TIVAP and diagnosis of thrombosis was 35.5 days (6 - 292 days). Diffuse subcutaneous edema in the ipsilateral UE was diagnosed on UE-CT venograms in 13 patients, and edema was not detected on CT in two patients. All symptomatic patients had thrombosis in the axillary vein on CT images. Symptoms were improved in all treated patients including removal of TIVAP at the time of diagnosis and following anticoagulation therapy (Figure 1). In the 18 symptomatic patients, tip locations were from the distal third of the superior vena cava to the right atrium in 16 patients and in the superior vena cava in two patients. The mean BMI value was 23.1. Among the patients with reported smoking status, nine patients were ex-smokers and one was a smoker. Metastatic lesions at the time of TIVAP insertion were detected in 10 patients. Based on the Khorana model, five patients were at high risk, seven patients at intermediate risk, and six patients at low risk.
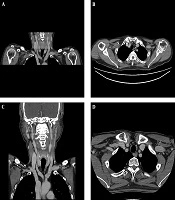
A 64-year-old male with hypopharyngeal cancer. Coronal reformatted (A) and axial (B) images of the initial upper extremity-computed tomography (UE-CT) venogram show thrombosis in the right axillary vein;Coronal reformatted (C) and axial (D) images of follow-up neck CT obtained after 1 year show resolved thrombosis with treatment including explantation of the totally implanted venous access port (TIVAP) and anticoagulation therapy.
5. Discussion
In the present cohort study, the incidence of symptomatic axillary vein thrombosis was 0.38% and lower than previously reported rates. Evaluation of TIVAP-related complications in cancer patients showed that the rate of venous thrombosis was 2.1% - 12.8% in recent studies (9, 12-16). Piran et al. (14) reported that the rate of symptomatic venous thrombosis was 4.5%; all implanted ports were inserted via the internal jugular vein without mention of catheter size. Suleman et al. (15) reported the rate of TIVAP-related venous thrombosis was 1.29% after implantation of the TIVAP via the internal jugular vein with 8F-anti-thrombogenic polymer catheter. Tabatabaie et al. (16) reported that the rate of TIVAP-related venous thrombosis was 1.81% (926/51,049); however, information was not provided regarding the diameter and material of the catheter or access route. Yukisawa et al. (17) reported that the rate of TIVAP-related venous thrombosis was 73% (67/92) based on venous duplex sonography. In addition, 11% of the patients (10/92) showed obstruction of venous flow that required anticoagulation therapy, and symptomatic venous thrombosis was observed in 5.4% of the patients (5/92). Suggestive causes for the lower incidence of venous thrombosis in the present study compared with previous reports include underestimation of thrombosis due to inclusion of only symptomatic thrombosis, smaller catheter diameter, less traumatic puncture technique using a micropuncture needle under ultrasound guidance, evaluation of pre-procedural imaging, and the method of tip location (8, 18). A larger catheter diameter could produce more endothelial damage, resulting in a higher incidence of thrombosis (18). We speculate that these points could decrease the incidence of venous thrombosis in the present study.
Among the many types of malignancies, pancreatic and lung cancers were reported as significant predictors of venous thrombosis and catheter-related thrombotic events (19, 20). Both cancers were associated with significantly higher incidence of thrombosis after placement of the TIVAP in the present study compared with other cancer types. From the multiple binary logistic regression analysis, pancreatic cancer and lung cancer were statistically significant risk factors of symptomatic axillary vein thrombosis, which is the same as in previous reports.
In the present study, the median time between insertion of a TIVAP and diagnosis of thrombosis was 35.5 days (6 - 292 days). Piran et al. (14) reported a median diagnosis time of 103 days (13 - 371 days), while Tabatabaie et al. (16) reported a median diagnosis time of 95.5 days (35 - 244 days). Dridi et al. (5) reported a mean diagnosis time of 56 days and 48% occurred within the first 2 months. Yukisawa et al. (17) reported a median diagnosis time of 37.2 days (11 - 77 days). In the present study, 50% of patients (9/18) were diagnosed within 1 month.
The Khorana risk score is a validated tool for estimation of venous thrombosis during chemotherapy (21). Hohl Moinat et al. (20) reported that a high Khorana risk score was a significant predictor of venous thrombotic events and catheter-related thrombotic events. In the present study, five patients were at high risk, seven patients at intermediate risk, and six patients at low risk based on the Khorana score. The Khorana risk score did not appropriately stratify patients at risk of symptomatic venous thrombosis.
The present study had several limitations. First, due to the retrospective nature of the review, some data were not accessible. Follow-up of CT images was inhomogeneous. Patient comorbidities, chemotherapy regimens, evidence of radiotherapy and surgery, and medication such as antithrombotic drugs, were not evaluated, which could introduce bias for evaluating venous thrombosis. Furthermore, cumulative survival analysis was not performed due to the small number of symptomatic events and lack of some data. Further prospective and well-organized studies are needed to provide precise and reliable results. Second, the size of axillary and subclavian veins was not considered. The same catheter size and material were used for all patients. Considering the different size of veins in patients, determining the size of the axillary and subclavian veins on imaging modalities such as ultrasound or CT could be important to evaluate the symptomatic axillary venous thrombosis in cancer patients with a TIVAP. Third, only symptomatic axillary venous thrombosis was investigated. Pulmonary embolism as a potential source of bias was not evaluated.
The current study shows that after insertion of TIVAPs through the axillary vein as a single incision technique, symptomatic axillary vein thrombosis rarely developed. Single incision technique for TIVAP implantation via the axillary vein seems to be safe with a low symptomatic thrombosis rate. Pancreatic cancer and lung cancer were associated with the risk of symptomatic axillary vein thrombosis.