1. Background
Budd-Chiari syndrome (BCS) is a group of disorders that are characterized as hepatic venous outflow tract obstruction, regardless of the mechanism of obstruction, which can be located at the level of the hepatic venules, the large hepatic veins and the inferior vena cava or the right atrium (1-3). Obstruction caused by cardiac and pericardial diseases and sinusoidal obstruction syndrome is not considered BCS (3, 4). The impaired hepatic venous flow caused by thrombosis or structural compression will lead to dramatically increased sinusoidal pressure, which results in hepatic venous congestion, ischemic injury, and eventually liver cirrhosis (1). Depending on the duration and severity of the disease, BCS can be categorized as fulminant (5%), acute (20%), and subacute or chronic BCS (60%) (2). In patients with fulminant or acute liver disease, venous collaterals have not yet been established, whereas several types of collateral circulation are seen in subacute or chronic forms in an attempt to decompress the hepatic sinusoidal pressure (1). The collateral circulation can be classified as intrahepatic, extrahepatic and portosystemic pathways (5). Currently, spontaneous portosystemic shunts (SPSS) in patients with BCS have not been sufficiently investigated by ultrasound. In this study, the ultrasonic features of spontaneous portosystemic shunts in patients with BCS were investigated.
2. Objectives
In this study, we aimed to assess the sonographic feature of SPSS in patients with BCS and to evaluate the differences in the main portal vein diameter among multiple types of portosystemic shunts.
3. Patients and Methods
3.1. Patients
All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the 1964 Declaration of Helsinki and later versions. Written informed consent from the patients was waived. Ultrasonograms of 352 chronic BCS patients from June 2000 to November 2015 were retrospectively studied. Diagnoses of BCS were confirmed via digital subtraction angiography (DSA) and ultrasonography (US). The only criterion for inclusion in this study was adequate visualization of spontaneous portosystemic shunts on ultrasonogram. However, some types of SPSS (such as coronary venous collaterals) that may not be adequately visualized by ultrasound due to excessive intestinal gas, ascites and technical difficulty were excluded from this study. All sonograms were acquired by an expert with more than 20 years of experience in ultrasound investigation and further evaluated by two expert sonographers with 7 and 10 years of experience with consensus agreement. Of these 352 patients, 44 patients were found to have SPSS, which were first detected by ultrasound and then confirmed via DSA, computed tomography angiography (CTA) or magnetic resonance venography (MRV) after 1 to 2 weeks.
3.2. Ultrasonic Examination
Ultrasonography was performed using an HDI 3500 (ATL-Philips, Bothell, WA, USA), an Envisor HD system (Philips Healthcare, Best, The Netherlands), or a Logiq E9 (GE Healthcare, Waukesha, WI, USA) with 3 to 6 MHz convex transducers. All examinations were performed early in the morning after patients had fasted more than 8 hours and all patients were scanned in the supine or left recumbent position. First, the courses of portal veins and splenic veins were carefully observed to determine whether there was collateral circulation (portosystemic venous shunts) between the portal veins and the inferior vena cava, hepatic veins, and accessory hepatic veins or between the splenic veins and the left renal veins. In the presence of SPSS, the diameter of the corresponding vessels was measured during normal respiration, and the venous shunts were classified. Doppler sonography was used to observe and record the blood flow direction and velocity within the portal veins. Doppler gain and wall filtering were optimized for the lumen without overpainting or aliasing. The Doppler angle of incidence was less than 60°, and the sample volume was adjusted to include at least one third of the lumen’s diameter. The mean velocity was calculated by integrating the time-averaged value based on the Doppler spectrum. The maximum diameter of the portal trunk and the mean portal flow velocity were measured three times, with the average values used for analysis.
3.3. Statistical Analysis
Continuous quantitative variables are expressed as the mean ± standard deviation or the median (first quartile-third quartile); whereas categorical variables are summarized as counts (percentages). Normality was tested using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) was performed to evaluate the difference in portal vein diameter, and multiple comparisons between the groups were performed using the LSD-t-test. P values < 0.05 were considered statistically significant. The statistical values were assessed using SPSS version 23.0 (IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY).
4. Results
4.1. Clinical Information of 44 BCS Patients with SPSS
The forty-four patients (18 male, 26 female) had a mean age of 46.3 ± 9.4 years, ranging from 27 to 65 years. Among all patients, the median time from the first clinical symptom to diagnosis was 36 months (first quartile-third quartile, 8 - 91 months). The main clinical symptoms were right upper abdominal distension, varicose veins on the abdominal wall and edema of the lower extremities. Five patients had a history of upper gastrointestinal bleeding, and 2 patients without definite symptoms were found during the health examination in our hospital. Clinical and demographic characteristics of the study patients are shown in Table 1.
4.2. The classifications of Spontaneous Portosystemic Shunts
SPSS were detected in 44 of the 352 BCS patients by ultrasound and confirmed by DSA, CTA, or MRV. According to the blood drainage patterns of SPSS, the 44 cases were classified as the following five types:
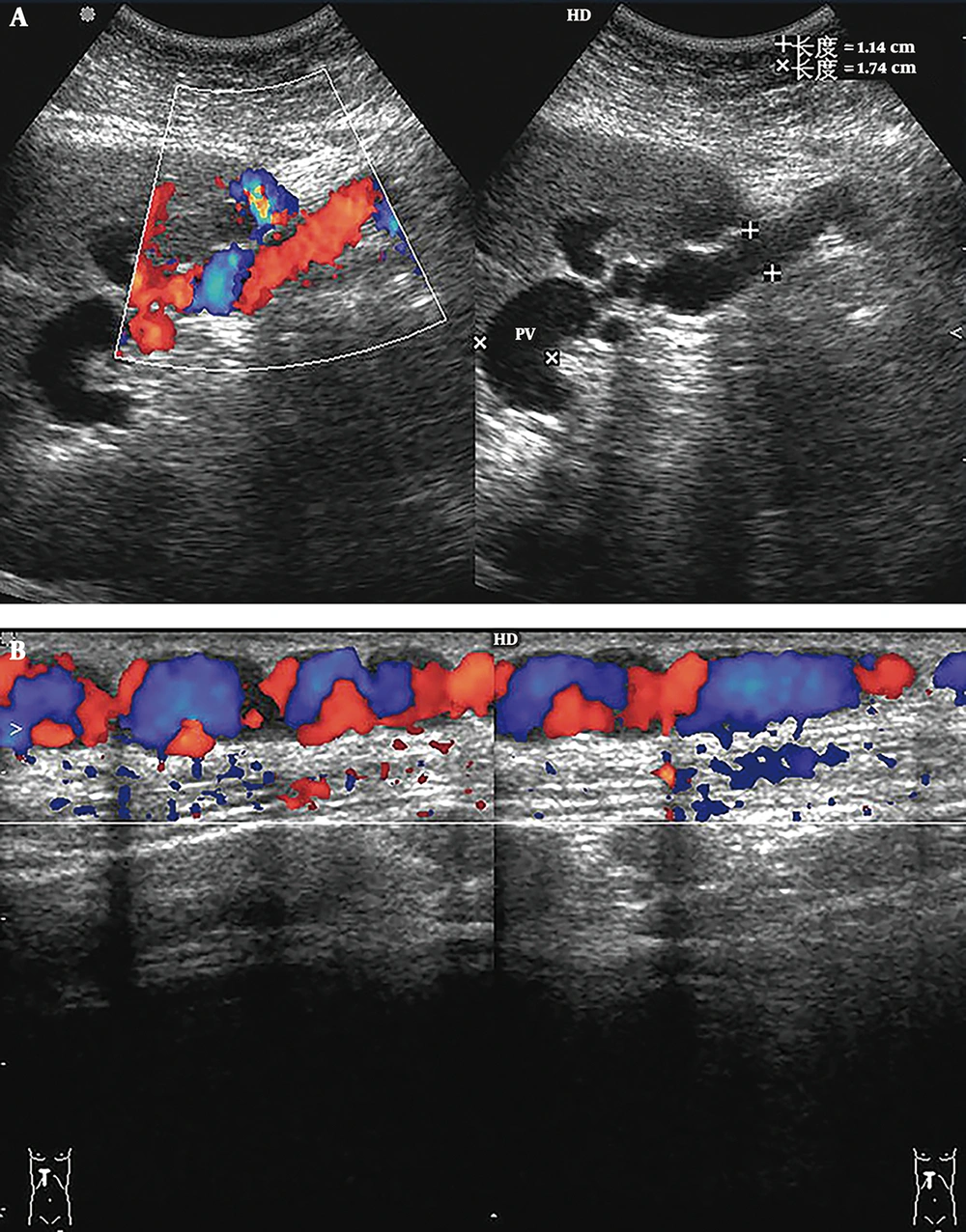
1) Portal-Umbilical Shunts (15 cases): The ultrasonic feature was that the umbilical veins were recanalized. Blood from the left sagittal branch of the portal vein flowed to the umbilical veins and then to the superficial periumbilical epigastric veins. The superficial periumbilical epigastric veins were tortuous and dilated (Figure 1). The mean diameter of 15 recanalized umbilical veins was 0.88 ± 0.18 cm, and the average velocity was 18.50 ± 3.21 cm/s; the average diameter of the main portal veins was 1.36 ± 0.16 cm. Among 15 patients, 14 exhibited hepatopetal blood flow in the main portal veins, with an average velocity of 12.71 ± 2.94 cm/s, and the rest exhibited hepatofugal blood flow, with a velocity of 6.22 cm/s.
Portal-umbilical shunt. A, Sonograms show that blood from the left sagittal branch of the portal vein flows to the recanalized umbilical vein. The umbilical vein is dilated, with a diameter of 1.14 cm; B, Color Doppler sonograms show tortuous and dilated superficial periumbilical epigastric veins.
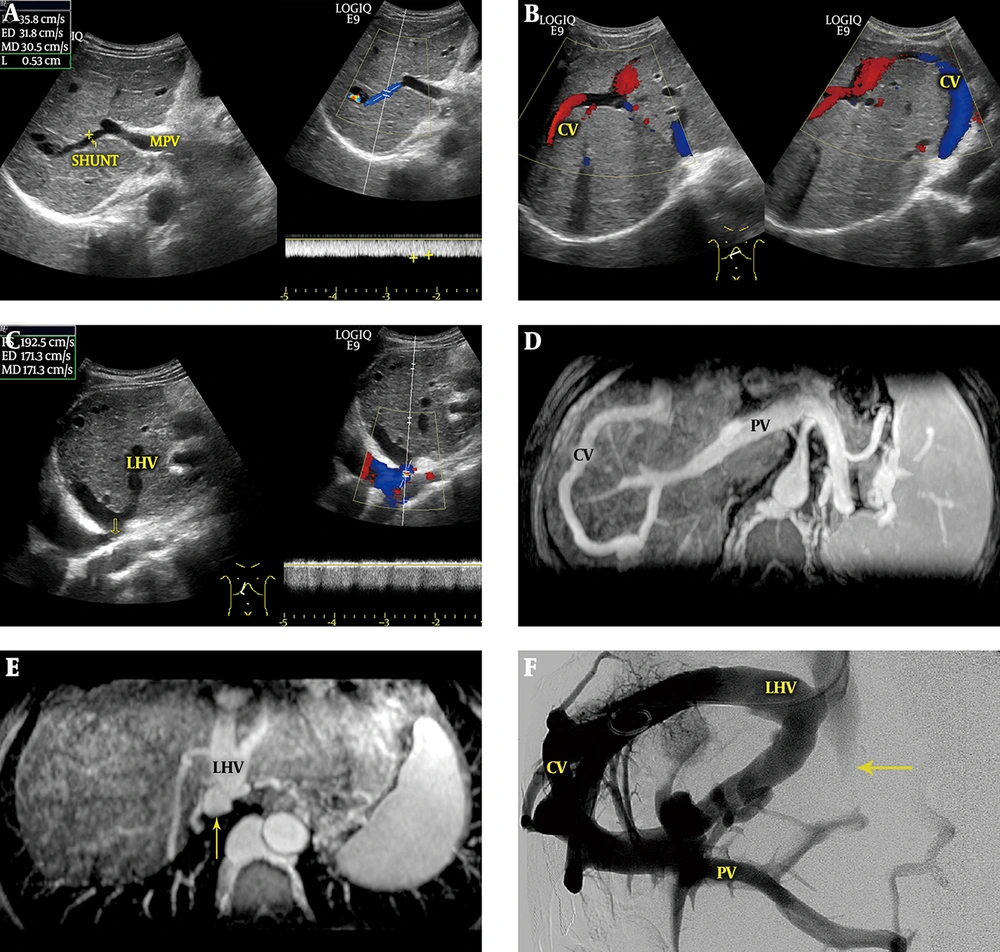
2) Portal-Hepatic Shunts (11 cases, including six cases with portal vein-left hepatic vein shunts, four cases with portal vein-right hepatic vein shunts, and one case with portal vein-middle hepatic vein shunts): Sonographic findings revealed that the portal venous blood flowed through single or multiple communications into the hepatic veins mentioned above or onward to the other intercommunicating hepatic vein, and finally into the inferior vena cava (IVC) (Figure 2); the average diameter of the main portal veins was 1.29 ± 0.15 cm. Among 11 patients, 9 exhibited hepatopetal blood in the main portal veins, with an average velocity of 19.30 ± 2.57 cm/s; 1 exhibited hepatofugal blood, with a velocity of 7.13 cm/s; and the rest exhibited hepatopetal and hepatofugal blood simultaneously.
Portal-hepatic shunt. A, Sonograms show that the blood of the main portal vein (MPV) flows to the communicating vessels (CVs) via the shunt. The diameter of the shunt is 0.53 cm, and the mean flow velocity is 30.5 cm/s; B, Sonogram shows the intrahepatic CVs from the shunt to the left hepatic vein (LHV); C, Sonogram shows the inlet of the LHV, with an accelerated mean flow velocity of 171.3 cm/s. Membranous stenosis is also found in the inlet (arrow); D, Magnetic resonance venography (MRV) shows that blood from the portal vein (PV) flows to the CVs via the shunt; E, Blood from the CVs drained into the inferior vena cava (arrow) via the LHV; F, Venography following placement of the guidewire in the LHV illustrates the presence of a portal-hepatic venous shunt and shows complete occlusion of the retrohepatic segment of the inferior vena cava (arrow).
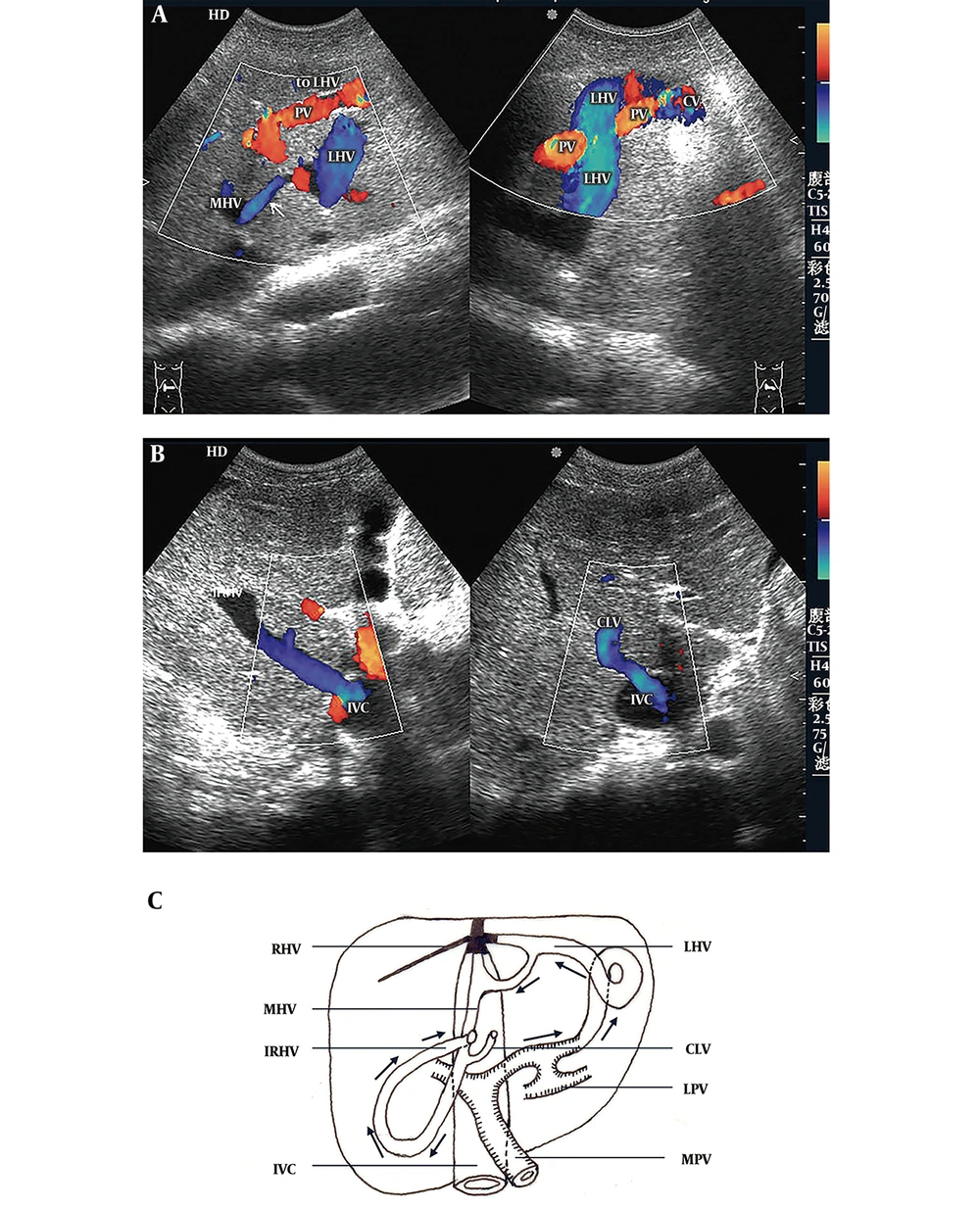
3) Portal-Accessory Hepatic Shunts (the accessory hepatic veins include the inferior right hepatic vein and caudate lobe vein) (six cases, including three cases with portal vein-inferior right hepatic vein shunts, two cases with portal vein-caudate lobe vein shunts and one case with portal vein-inferior right hepatic vein + caudate lobe vein shunts): Sonographic features were roughly consistent with those of portal-hepatic venous shunts. Portal venous blood flowed through the collateral circulation into the accessory hepatic veins and then into the inferior vena cava (Figure 3). The average diameter of the main portal veins was 1.23 ± 0.12 cm. All patients had hepatopetal blood flow in the main portal veins, with an average velocity of 16.30 ± 2.42 cm/s.
Portal-accessory hepatic venous shunt. A, Color Doppler sonogram shows that the blood from the portal vein (PV) flows to the left hepatic vein (LHV) through the communicating vessel (CV) (right hand image) and onward to the middle hepatic vein (MHV) (left hand image) via communicating branches (arrow); B, Color Doppler sonograms show that blood drains into the inferior vena cava (IVC) by the dilated inferior right hepatic vein (IRHV) (left hand image) and caudate lobe vein (CLV) (right hand image); C, Diagram of a portal-accessory hepatic shunt shows segmental occlusion of the LHV, MHV, and IVC, and complete occlusion of the right hepatic vein (RHV). Blood from the left portal vein (LPV) flows into the LHV and MHV through the CVs and then drains into the IVC through the IRHV and CLV.
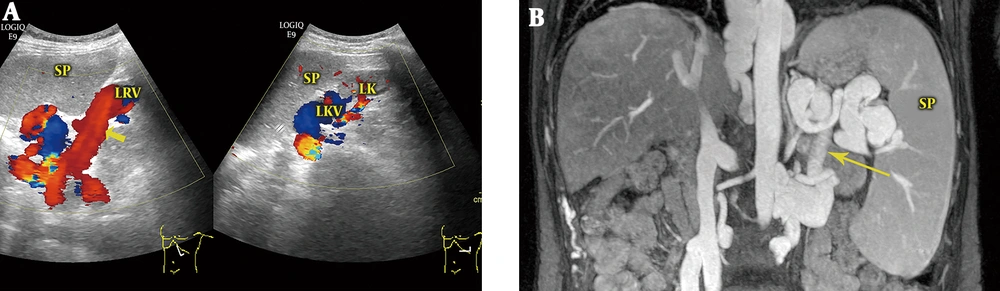
4) Splenorenal Shunts (Eight Cases): The average diameter of the main portal veins was 1.12 ± 0.14 cm. The splenic veins at the splenic hilus were dilated and tortuous, with an average diameter of 1.41 ± 0.16 cm. Hepatofugal main portal venous blood flow was found in all eight patients, with an average velocity of 7.0 ± 1.27 cm/s. Retrograde blood flow was also found in the splenic vein posterior to the pancreas. Multiple-section scanning revealed that the branches of the splenic veins anastomosed with the left renal veins through communicating branches (Figure 4). Color Doppler showed that the blood within the main portal veins reversed to the splenic veins and then continued to the left renal veins via communicating branches.
Splenorenal shunt. A, Color Doppler sonogram shows that the splenic venous branches anastomose with the left renal vein (LRV) through communicating branches (arrow) and that the blood from the splenic vein flows to the LRV; B, Magnetic resonance venography (MRV) shows that the splenic vein anastomoses with the LRV via the communicating vein (arrow). SP, spleen; LK, left kidney; LKV, left kidney vein.
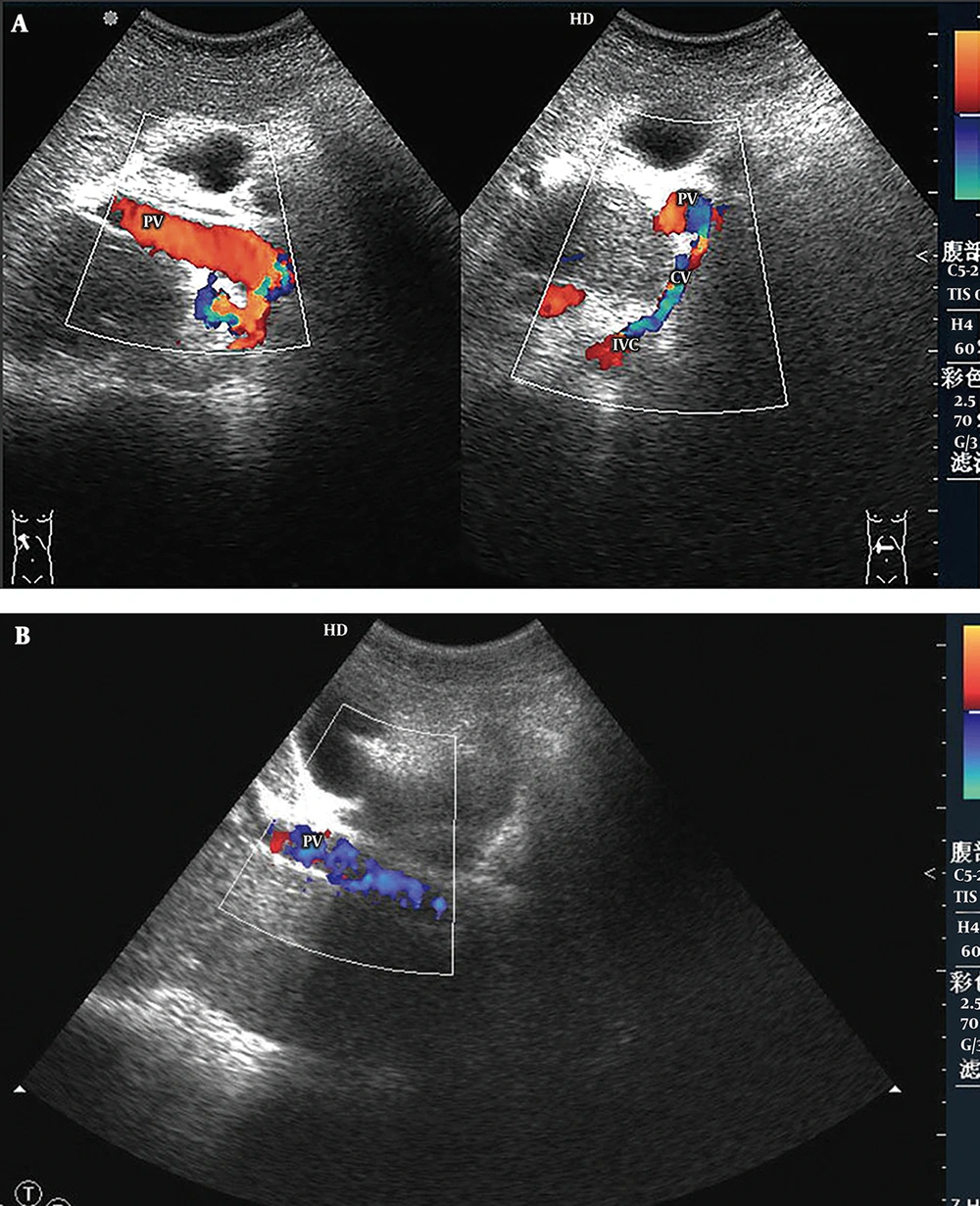
5) Main Portal Vein-Inferior Vena Cava Shunts (Four Cases): Abnormal directional flow was detected in all patients. Among them, hepatofugal blood in the main portal veins was found in three patients, and bidirectional (hepatopetal and hepatofugal) blood flow was found in one patient. Hepatofugal blood in the main portal veins flowed into the inferior vena cava through the collateral circulation (Figure 5). The main portal veins were obviously dilated, with an average diameter of 1.60 ± 0.16 cm. The portal hemodynamic characteristics of the 44 BCS patients are shown in Table 2.
Main portal vein-inferior vena cava shunt. A, Color Doppler sonogram shows part of the hepatopetal blood flow on the longitudinal section (left hand image) and bidirectional blood flow on the cross section of the portal vein (PV) (right hand image). The hepatofugal blood in the portal vein flows to the inferior vena cava (IVC) via the communicating vessel (CV); B, In another patient, color Doppler sonogram demonstrates a hepatofugal flow in the main portal vein.
| Items | Portal-UV shunts (n = 15) | Portal-HV shunts (n = 11) | Portal-AHV shunts (n = 6) | Splenorenal shunts (n = 8) | MPV-IVC shunts (n = 4) |
|---|---|---|---|---|---|
| The direction and velocity of MPV flow, cm/s | |||||
| Hepatopetal | 14 (12.71 ± 2.94) | 9 (19.30 ± 2.57) | 6 (16.30 ± 2.42) | 0 | 0 |
| Hepatofugal | 1 (6.22) | 1 (7.13) | 0 | 8 (7.0 ± 1.27) | 3 (-) |
| Bidirectional | 0 | 1 (-) | 0 | 0 | 1 (-) |
| Mean diameter of the MPV, cm | 1.36 ± 0.16 | 1.29 ± 0.15 | 1.23 ± 0.12 | 1.12 ± 0.14 b, c | 1.60 ± 0.16b, c, d, e |
Portal Hemodynamic Characteristics of the 44 BCS Patients and Multiple Comparisons of the MPV Diameter Among Different Types of SPSSa
No combined shunts were detected in the 44 BCS patients. Dilated hepatic arteries with accelerated blood flow were detected by ultrasound in all three patients with hepatofugal or bidirectional blood flow of the main portal veins in the first two types. Main portal vein-inferior vena cava shunts had a significantly larger mean portal trunk diameter compared with all other shunt types (P < 0.05 for all comparisons). In addition, the mean portal trunk diameters in portal-umbilical shunts and portal-hepatic shunts were obviously larger than that of splenorenal shunts (P < 0.05), while there were no statistically significant differences between the other types. Statistical analysis results are reported in Table 2.
4.3. The Other Major Sonographic Findings in 44 Patients with Spontaneous Portosystemic Shunts
Among the 132 total hepatic veins of 44 patients in this group, there were only 13 hepatic veins with unobstructed lumens, among which there were five hepatic veins with tortuous lumens caused by the liver parenchyma extrusion that remained. There were 119 abnormal hepatic veins (including stenosis and occlusion of the lumen), accounting for 90.2% (119/132) of all hepatic veins, with an average of 2.7 (119/44) per patient. Except for the 58 lumens that were stenosed to different degrees, there were 58 lumens with complete occlusion and 3 lumens that had disappeared due to thrombosis, accounting for 51.3% of the veins (61/119). Among 44 patients, there were only 4 with a patent inferior vena cava (IVC), while the remaining 40 patients had mixed BCS, in which obstructed HVs and an obstructed IVC co-existed in the same patient. Collateral pathways of the inferior vena cava were also detected in each patient with an obstructed inferior vena cava by DSA, CTA or MRV. The blood from the obstructed inferior vena cava was drained into the superior vena cava via the ascending lumbar vein, left renal vein or left inferior phrenic vein, as previously reported (5, 6).
5. Discussion
In this group of patients with chronic BCS, portal-umbilical shunts caused by the reopened umbilical vein were the most common type and the incidence of patent paraumbilical vein was 4.3% (15/352). Sonograms showed that the recanalized umbilical vein runs from the umbilical part of the left branch of the portal vein to the liver margin, and drains into the anterior abdominal veins. Clinically, this route is characterized by a “Medusa’s head” appearance, i.e., a network of dilated veins around the umbilicus (7). Because the course of the umbilical vein is roughly towards the transducer, maintaining a low angle (< 60°), this shunt can be accurately diagnosed with a transabdominal probe.
Previous studies have confirmed that few communicating branches exist between portal veins and hepatic veins in normal individuals (8). Among patients with chronic BCS, portal hypertension due to hepatic venous outflow tract obstruction is found in almost all patients. The increasing pressure in the portal vein makes the small communicating branches gradually dilate and eventually develop into enlarged collaterals, namely, portosystemic shunts. Therefore, a large amount of portal vein blood can be drained through the intrahepatic portosystemic venous shunts rather than the hepatic sinusoid, directly into the hepatic vein and accessory hepatic vein (the caudate lobe vein or inferior right hepatic vein), and then enter the systemic circulation. The spontaneous shunts between the portal vein and hepatic vein, as well as the accessory hepatic vein, are similar in terms of hemodynamics and ultrasonography; i.e., the left or the right branch of the portal vein connects the hepatic vein or accessory hepatic vein via communicating branches to the inferior vena cava. In accordance with our previous report regarding the high incidence of hepatic vein obstruction in BCS patients, dilated collateral vessels between the hepatic vein and hepatic vein or accessory hepatic vein were observed in most patients (9, 10). Therefore, portal-hepatic and portal-accessory hepatic portosystemic shunts would easily be mistaken as the collateral vessels mentioned above. Finding the communication vessel directly connecting the branches of the portal vein to the hepatic vein or accessory hepatic vein is the key to the diagnosis of the two types.
By observing multiple real-time sections, we found that the communicating branches of the splenic vein anastomose with the left renal vein in the retroperitoneal space, which is the direct sign needed to diagnose spontaneous splenorenal shunts. However, there are numerous dilated and tortuous vessels in the region of the splenic and left renal hilum, and they are sometimes difficult to distinguish from the splenorenal shunt (11). Observation of the hepatofugal blood flow of the portal vein and splenic vein posterior to the pancreas, as well as the dilated left renal vein, was helpful for determining the existence of this type of shunt.
Spontaneous main portal vein-inferior vena cava shunts in BCS have rarely been reported in the literature. There was only one report of a spontaneous direct portacaval shunt between the right portal vein and the inferior vena cava. However, the blood flow volume of this spontaneous shunt was too small to provide sufficient hepatic decompression (12). Although all the patients with this type have communication vessels between the main portal vein and inferior vena cava, the communicating vessels were also small and failed to reduce the pressure of the portal vein efficiently.
The current study showed that there were 119 obstructed hepatic veins in 44 patients, accounting for 90.2% of all hepatic veins, with an average of 2.7 (119/44) abnormal hepatic veins per patient, which is consistent with our previous research (9, 10). However, there were 61 veins with complete lumen occlusion among 119 abnormal veins (including 58 veins with lumen occlusion and three veins with lumen occlusion caused by thrombus formation), accounting for 51.3% of abnormal hepatic veins (61/119), which was far higher than the frequency reported in a previous study involving 180 veins with lumen occlusion among 542 abnormal hepatic veins (33.2%, 180/542) (9). For this reason, more extensive occlusion of the hepatic veins may aggravate portal hypertension. The postsinusoidal portal hypertension caused by obstruction of the hepatic vein may be the basis of the spontaneous formation of portosystemic shunts. Although all 36 patients in this group had inferior vena cava obstruction, their collateral pathways could sufficiently reduce the inferior vena cava pressure. Therefore, there was still a large pressure difference between the portal vein and inferior vena cava, which may be one of the reasons for the formation of SPSS in this group.
Dilation of the main portal veins among the former four types mentioned above was smaller than that of the fifth type. In addition, the former three types not only had the presence of large portosystemic communicating branches, but the velocity of hepatopetal portal venous flow did not decrease obviously. Although all the main portal vein of the fourth type had hepatofugal flow, large communicating branches were simultaneously found between the splenic vein and left renal vein. In addition, hepatofugal or bidirectional portal venous flow was exhibited in three patients with the first two types. All of these patients had a dilated hepatic artery with accelerated blood flow, which may be seen as a result of portal venous compromise in portal hypertension. The above ultrasonic features illustrated that the amount of shunted blood in the former four types was larger. Therefore, for the first four types of portosystemic shunts, we can mainly treat the lesions of the hepatic vein and inferior vena cava, whereas the last type requires a transjugular intrahepatic portosystemic shunt (TIPS) due to inability to provide sufficient hepatic decompression (12).
In summary, sonographic examination is a reliable way to observe and record the course, location and hemodynamics of the portosystemic venous shunts, which can be helpful for establishing therapeutic plans.