1. Background
Focal seizures are the most common type of seizures, accounting for almost 60% of all seizures. The most common form of focal seizures is temporal lobe epilepsy (TLE) (1). TLE was first categorized by the International League Against Epilepsy (ILAE) under the group of localization-related symptomatic epilepsy in 1989 (2). There are few epidemiological studies on TLE, and the reports are controversial (1, 3-5). TLE is subcategorized as mesial temporal (amygdalohippocampal) and lateral temporal lobe epilepsy (LTLE) or neocortical (6). In 2010, the ILEA identified mesial temporal lobe epilepsy (MTLE) as a subclass of limbic epilepsies. The MTLE was defined as a symptomatic focal epilepsy. The pathophysiology of this condition is usually hippocampal sclerosis (HS) (2, 7).
TLE is diagnosed according to the patient’s history of focal seizure symptoms and is supported by electroencephalography (EEG), showing a temporal lobe epileptiform-like activity, and by imaging of the lesion (8). Commonly, TLE does not respond well to antiepileptic drugs. It is the most common form of medically intractable epilepsy (MIE), responsible for most epilepsy surgeries with a good prognosis (9). To localize the epileptic site for surgery, different neuroimaging methods can be applied. These methods include structural magnetic resonance imaging (MRI) to show the temporal lobe anatomy and the presence of tissue sclerosis, diffusion-weighted imaging, diffusion tensor imaging, magnetoencephalography, functional MRI (fMRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT), all of which can improve the outcomes of surgery (10, 11).
Perfusion MRI is one of the modalities used to evaluate the cerebral hemodynamics and localize intracranial neoplasia and cerebrovascular events. Perfusion imaging is divided to non-contrast methods (arterial spin labeling) and contrast-enhanced methods. The contrast-enhanced methods are subdivided into dynamic contrast-enhanced (DCE) T1W imaging and dynamic susceptibility contrast (DSC) T2 * W imaging (12, 13). DSC-MRI is the method of choice to measure the brain blood perfusion parameters, as it relies on the T2* signal loss of gadolinium-based contrast bolus, passing through a capillary bed. The most commonly calculated parameters are the relative cerebral blood volume (rCBV), relative cerebral blood flow (rCBF), mean transit time (MTT), and time to peak (TTP) (12-14).
Studies have shown that there may be a significant relationship between TLE and perfusion of the epileptic site. They have investigated different aspects of perfusion changes in epilepsies, especially localized epilepsies, such as TLE (15-21). One of the most inspiring studies was conducted by Xing et al., which demonstrated a decrease in the perfusion parameters in the epileptic side of TLE patients compared to the opposite side (19). However, there are limited studies evaluating changes in the cerebral blood flow perfusion parameters in epileptic patients, and there is no similar study on Iranian patients.
2. Objectives
In this study, we aimed to evaluate the blood perfusion parameters, including rCBV and rCBF, in patients diagnosed with MTLE, using DSC perfusion MRI to determine whether there is a change in these parameters and whether it can be used as a novel alternative method for localizing the epileptic side.
3. Patients and Methods
Twenty-two patients (mean age: 26.45 ± 6.87 years), who were diagnosed with TLE clinically and electrophysiologically by a neurologist, were studied. All patients had previous MRI scans, indicating unilateral MTLE as sclerosis and volume loss, with no other lesions, such as tumors or cerebrovascular disease (as the exclusion criteria). All patients gave their informed consent about the study procedures, and the ethics committee of the university approved the procedures.
MRI of patients was performed using a 1.5 T MRI scanner (MAGNETOM Aera; Siemens, Erlangen, Germany) and a 12-channel phased-array matrix head coil. The applied protocol included the following sequences: (1) axial T2-weighted images (T2WI) with repetition time (TR)/echo time (TE) of 3500/125 ms, field of view (FOV) of 230, and matrix size of 384 × 256; (2) axial T1-weighted images (T1W1) with TR/TE of 600/26, FOV of 230, and matrix size of 320 × 256; coronal 3D fluid attenuated inversion recovery (FLAIR) with TR/TE of 5000/415 ms, inversion time (TI) of 1800 ms, and isotropic pixel size of 0.9 mm; (3) coronal T1W MP-RAGE with TR/TE/TI of 2000/3/1000 ms and 1-mm isotropic pixel size.
The DSC-MRI images were acquired using a T2*-weighted gradient-echo, echo-planar imaging (GRE-EPI) sequence with 50 continuous phases. The contrast agent (gadoterate meglumine, DOTAREM) bolus was administered in the 10th phase at 0.2 mmol/kg body weight dose, followed by 20 mL of saline flush. The contrast medium was injected by a MEDRAD injector (Liebel-Flarsheim Co., Cincinnati, OH, USA) at an injection rate of 4 mL/sec. Another axial gradient-echo T1W sequence with the same parameters, such as FOV, slice thickness, and coverage, was obtained after perfusion imaging for a better localization of the region of interest (ROI).
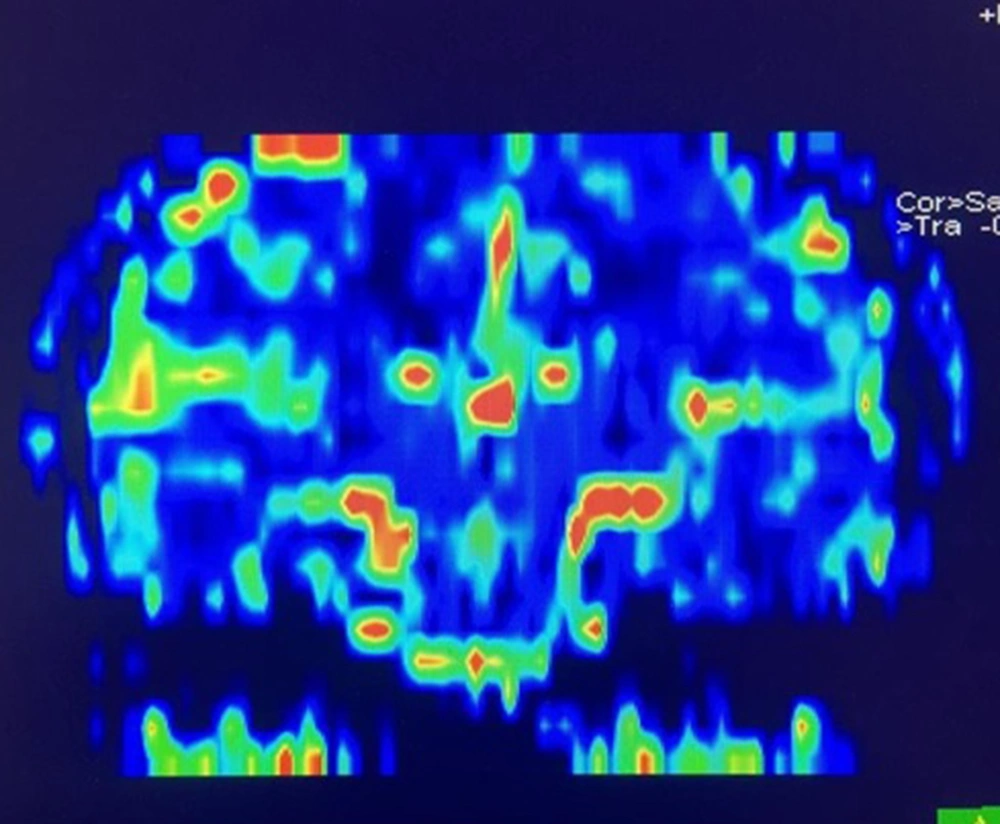
The perfusion images were processed by the Siemens perfusion software, and the rCBV and rCBF maps were generated based on the gamma variate fit. Since the perfusion differences were in the range of < 10%, and the hippocampus could be practically visualized in few axial slices, we used a quantitative analysis. Besides, a coronal reconstruction of the rCBV map in one of the patients in presented in Figure 1.
For the quantitative analysis, a single neuroradiologist with 15 years of experience, who was blinded to the clinical data of the patients, placed the ROI on the hippocampus and the parahippocampal gyrus. The free-shaped ROI with a mean size of 15 pixels was placed on the T1W image, which showed the hippocampus the best, and was copied at the same level of DSC images. After determining the CBV and CBF values relative to the ROI of each side, the asymmetry index (AI) was calculated by Equation 1 (19):
The DSC MRI-derived perfusion parameters were statistically analyzed in SPSS version 20.0 (IBM, SPSS Inc., USA). The normal distribution of parameters in DSC-MRI was assessed using Kolmogorov-Smirnov test. The measured rCBV and rCBF were examined using paired two-sample t-tests to determine differences in the perfusion parameters (significance threshold, P < 0.05).
4. Results
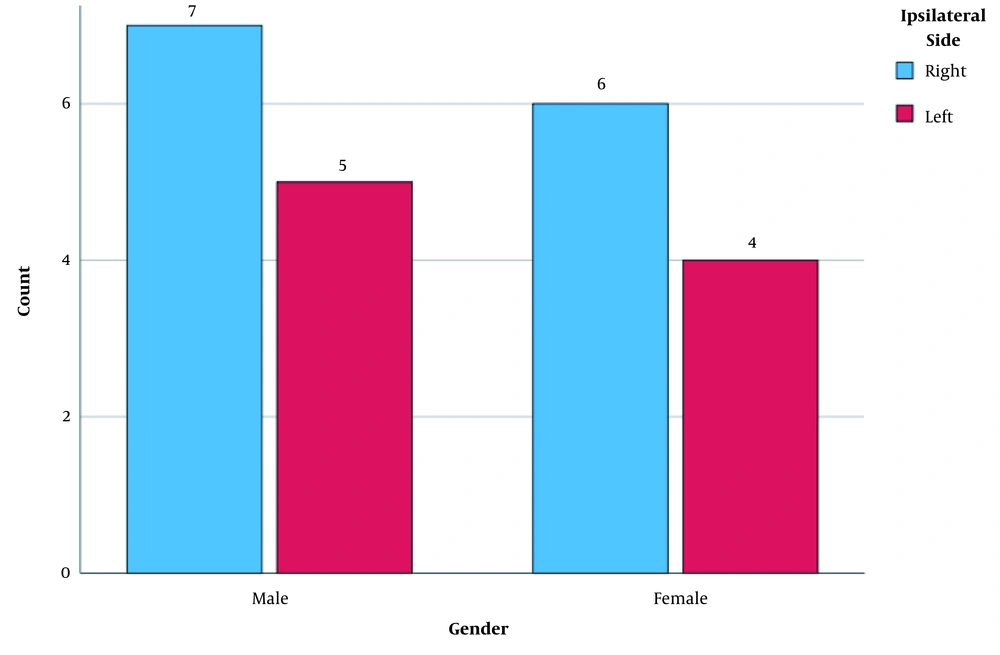
A total of 22 patients were enrolled in this study, including 12 (54.4%) males and 10 (45.5%) females. Nine patients (40.9%) had left-sided TLE, and 13 patients (59.1%) had right-sided TLE. No significant difference was observed in the seizure side between males and females (P = 0.93) (Figure 1). The mean (standard deviation [SD]) age of the participants was 26.45 (6.87) years (range: 16 - 45 years), and the mean (SD) age of the patients with right- and left-sided TLE was 26.23 (7.16) and 26.78 (6.79) years, respectively. No significant difference was found in terms of age between the two groups (P = 0.85). The demographic data of the participants are shown in Table 1.
| Patients | Gender | Age (y) | Epileptic side | AI rCBV | AI rCBF |
|---|---|---|---|---|---|
| 1 | Male | 18 | Right | 9.76 | 9.93 |
| 2 | Female | 16 | Right | 8.24 | 8.93 |
| 3 | Male | 34 | Right | 9.74 | 10.28 |
| 4 | Female | 21 | Right | 6.22 | 10.46 |
| 5 | Male | 32 | Right | 5.61 | 9.06 |
| 6 | Female | 27 | Right | 8.17 | 17.88 |
| 7 | Female | 25 | Right | 8.16 | 6.99 |
| 8 | Female | 33 | Right | 6.17 | 8.34 |
| 9 | Male | 17 | Right | 11.63 | 10.05 |
| 10 | Male | 38 | Right | 5.46 | 9.85 |
| 11 | Male | 26 | Right | 8.51 | 8.07 |
| 12 | Female | 32 | Right | 5.53 | 8.08 |
| 13 | Male | 22 | Right | 7.73 | 8.37 |
| 14 | Female | 41 | Left | 4.43 | 6.25 |
| 15 | Male | 24 | Left | 2.60 | 7.34 |
| 16 | Male | 28 | Left | 2.52 | 6.61 |
| 17 | Female | 24 | Left | 3.58 | 7.34 |
| 18 | Male | 19 | Left | 5.14 | 12.32 |
| 19 | Female | 19 | Left | 6.43 | 9.06 |
| 20 | Female | 32 | Left | 3.69 | 6.79 |
| 21 | Male | 26 | Left | 3.26 | 5.26 |
| 22 | Male | 28 | Left | 4.03 | 4.54 |
Abbreviations: AI, assymetry index; rCBV, relative cerebral blood volume; rCBF, relative cerebral blood flow.
The results showed that the mean (SD) rCBV was 1.66 (0.29) on the right side (range: 1.24 - 2.21) and 1.88 (0.34) on the left side (range: 1.05 - 2.35) in all patients. Besides, the mean (SD) CBF was 79.30 (16.55) on the right side (range: 51.4 - 112.3) and 89.90 (0.21.61) on the left side (range: 41.6 - 118.6). Moreover, the mean (SD) rCBV and rCBF in the affected side (nine left-sided and 13 right-sided) were 1.55 (0.24) and 70.09 (12.59), respectively. For the non-affected side, the mean (SD) values of rCBV and rCBF were 1.99 (0.26) and 99.10 (13.57), respectively.
In patients with epilepsy arising from the right side of the brain, the rCBV of the right side was significantly lower than the rCBV of the left side (P < 0.0001). Similarly, the right rCBF was lower than the left rCBF, and the difference was significant (P < 0.0001). Overall, the mean values of parameters for the right side of the brain were lower than those of the left side in patients with epilepsy affecting the right side (P < 0.0001). Also, similar analyses were performed in patients with epilepsy of the left side, which indicated similar results (P < 0.0001) (Table 2). In other words, in patients with epilepsy of the left side, both parameters were significantly lower in the left side as compared to the right side. Overall, the mean values of rCBV and rCBF were significantly lower in the affected side compared to the opposite side (Table 3).
| Variables | Epilepsy of the right side | Epilepsy of the left side | P-value |
|---|---|---|---|
| rCBV | P < 0.0001 | ||
| Right rCBV | 1.54 ± 0.20 | 1.84 ± 0.31 | |
| Left rCBV | 2.10 ± 0.17 | 1.58 ± 0.31 | |
| rCBF | P < 0.0001 | ||
| Right rCBF | 70.36 ± 10.38 | 92.21 ± 15.49 | |
| Left rCBF | 103.87 ± 10.83 | 69.71 ± 16.55 |
Abbreviations: rCBV, relative cerebral blood volume; rCBF, relative cerebral blood flow.
a Values are expressed as mean ± SD.
| Variables | Affected side | Non-affected side | P-value |
|---|---|---|---|
| rCBV | 1.55 ± 0.24 | 1.99 ± 0.26 | P < 0.0001 |
| CBF | 70.09 ± 12.59 | 99.10 ± 13.57 | P < 0.0001 |
Abbreviations: rCBV, relative cerebral blood volume; rCBF, relative cerebral blood flow.
a Values are expressed as mean ± SD.
The AI for both rCBV and rCBF was also measured for all patients listed in Table 1. The CBV and CBF were significantly different between the two sides of the brain in patients with unilateral epilepsy. Also, the positive values indicated that the parameters were lower in the affected side as compared to the opposite side. Besides, the frequency of epileptic side by gender is shown in Figure 2.
5. Discussion
In the present study, 22 patients with MTLE were examined, and the blood perfusion parameters were compared between the involved and healthy sides of the brain hemispheres. The results showed that the rCBV and rCBf were significantly lower in the affected side of patients with TLE, while these parameters were normal in the non-affected cerebral hemisphere. Comparison of the AI values also showed lower blood perfusion parameters in the affected side. Our findings highlighted the association between blood perfusion and TLE.
Some previous studies have investigated the role of brain blood perfusion in patients with epilepsy. In a study by O’Brien et al. in 2007, ten patients with TLE and ten controls were evaluated using MR contrast-enhanced perfusion imaging (MR-CEPI) and diffusion-weighted imaging (DWI). They found that patients with TLE had lower blood perfusion parameters. Based on their results, the CEPI and DWI could be reliable tools for non-lesion TLE (18); these findings are in line with our results.
In another study by Xing et al. in 2013, a total of 20 interictal cases of TLE and 20 healthy volunteers underwent conventional and DSC MRI. They reported similar results and showed that both rCBF and rCVB were lower in the ipsilateral side, compared to the healthy control subjects. Although our results are similar, both studies lack a large study population. Further research is needed on a larger population, including LTLE patients so that we can strongly recommend DSC-MRI for localizing the epileptic side (19). Moreover, Kim et al. performed a study on 65 patients with intractable mesial temporal epilepsy (MTE). Also, subtraction ictal SPECT co-registered to MRI indicated hypoperfusion in the affected areas of patients with MTE (21).
Moreover, in a study by Oner et al. in 2015, a total of 36 patients with TLE and 11 healthy volunteers were enrolled. Measurements of different parameters showed that in TLE patients, the rCBF ratio of the affected side was significantly lower than the unaffected side. They also emphasized that physicians could localize the affected area in TLE by using rCBF (22); these results are consistent with our findings. However, the key point of our study is that we measured both rCBV and rCBf in the patients and compared the AI values.
In another study by Gelfand et al., changes in the perfusion pattern of the temporal lobe were found in patients with TLE. They also suggested that these abnormalities might be due to atypical vascular distributions; however, further studies are needed in this area (23). Another study by Pizzini et al. from Italy confirmed the effectiveness of pulsed arterial spin labeling (PASL) MRI in diagnosis and lateralization of hypoperfusion in patients with TLE (24). This issue was also discussed by Cleeren et al. in 2015, who showed that different temporal lobe seizures occurred in a common network induced by epileptogenic factors, such as blood perfusion in the brain (25).
The main shortcomings of ASL imaging for TLE localization are the need for a strong magnetic field and limited perfusion parameters that ASL can produce. However, in the present study, by evaluating the difference between parameters in both sides of the brain, we attempted to show that the difference was significant and that DSC-MRI could be applied to localize the epileptic region. We believe that DSC imaging of the brain and comparison of AI values can be reliable tools for a more effective TLE localization, even with a clinical magnetic strength of 1.5 T. The MR perfusion imaging can be used instead of SPECT imaging for tracing physiological changes in the epileptogenic side due to non-ionizing radiation and more spatial resolution.
In conclusion, in patients with TLE, significantly lower blood perfusion parameters in the affected side of the brain can aid radiologists and neurologists to lateralize the MTLE region.