1. Background
Nephrolithiasis is a globally common disorder, affecting all populations regardless of geographic and socioeconomic factors (1). According to reports, 10% - 15% of people in Western countries experience nephrolithiasis during their lifetime, with an even higher risk in the Middle East (20% - 25%) (1, 2). Also, previous studies suggest a prevalence of 1.9% in the general population of Iran, especially in central and southern provinces (3, 4). There is a general consensus that a more extensive metabolic evaluation is needed for recurrent or high-risk stone formers. Patients with large stones are a subgroup of patients who need further metabolic evaluation via 24-hour urine collection (5).
Chronic nephrolithiasis is often considered the hallmark of a systemic disorder (6). The bone mineral density (BMD) is lower in renal stone formers, stemming from several proposed pathogeneses, including genetic factors, metabolic disorders (such as renal leak hypercalciuria), immunological processes, and dietary, environmental, and iatrogenic effects (7-10). A decrease in BMD has been shown to occur regardless of urinary calcium excretion, as observed in both normocalciuric and hypercalciuric patients (7, 11). This reduction in BMD is of great importance, since the overall risk of bone fracture is higher among nephrolithiasis patients as compared to the normal population (7, 12, 13).
Non-contrast computed tomography (CT) is universally recognized as the standard modality for detecting urinary stones. It has been applied in up to 71% of emergency department visits, substituting other imaging methods, such as X-ray and ultrasound (14). Guidelines provided by the American Urological Association (AUA) for the surgical management of urinary stones strongly emphasize the role of non-contrast CT scan as a preoperative assessment (15). Besides, data collection through CT imaging for urolithiasis can minimize the need for additional costs and radiation burden on patients (5). Studies conducted by Alacreu et al. (16) and Pickhardt et al. (17) established abdominal CT scan, compared to the universally standard DEXA scan, as a viable method for assessing BMD by measuring the Hounsfield units (HU) at different vertebral levels, specifically L1.
2. Objectives
We aimed to evaluate the relationship between the BMD of patients with urinary stones and the ancillary data, including stone diameter retrieved from CT images and urine biometrics in the 24-hour urinalysis. We also aimed to assess the role of non-contrast CT scan as a promising screening tool in these patients.
3. Patients and Methods
3.1. Patients and Setting
The sample population of this retrospective study included patients with kidney stones, undergoing percutaneous nephrolithotomy (PCNL) for stone removal, admitted to the urology ward of Shohada-e Tajrish Hospital, Tehran, Iran, from February 2015 to July 2019. The subjects were enrolled based on the inclusion criteria: (1) Having a predominant calcium component in the urinary calculi analysis; (2) having a non-contrast abdominopelvic CT scan a day before surgery; and (3) a complete 24-hour urine study collected at least one month after the PCNL procedure.
Of 390 PCNL patients referred to the ward in the target period, 148 patients met the inclusion criteria and were enrolled in the study. Each patient’s preoperative medical record was retrieved from the medical record database of Shohada-e Tajrish Hospital. The data included each subject’s basic information, 24-hour urine biomarkers, serum biochemical analysis, and preoperative abdominopelvic CT scan. Patients with no previous record of 24-hour urinalysis were excluded.
Urinalysis before the stone removal procedure was also considered as an exclusion criterion, since the urine biochemistry may be affected by larger stone burdens. Besides, patients receiving medical treatments for nephrolithiasis before surgery were excluded from the study to reduce outliers in the data. Patients receiving calcium or vitamin D3 supplements, as well as those with impaired parathyroid hormone (PTH) secretion, were also excluded from the study. According to the standard protocol (18), CT is routinely performed before all stone removal surgeries in our center; therefore, we did not include it in the criteria.
3.2. Radiological Analysis
CT imaging was performed using a 16-slice Somatom Sensation scanner (Siemens, Germany), calibrated daily to ensure the accuracy of attenuation measurements. A distinguished radiologist retrieved each subject’s CT data from the hospital database and analyzed the images, using an INFINITT PACS viewer, assessing bone mineral attenuation and calculating the urinary stone size. Stone size was defined as the maximum diameter of stones in either axial or coronal windows, measured in millimeters.
According to established standards for measuring bone mineral attenuation in CT images, vertebral attenuation was calculated on both coronal and axial CT cross-sections by designating an oval region of interest (ROI) with an approximate area of 2 cm2 at the L1 vertebra over the centermost area of the trabecular bone. Proper anatomical selection of the ROI was ensured by inspecting sagittal and lateral windows to avoid attenuation measurement distortions. Bone mineral attenuation was measured in Hounsfield units (HU). As established in previous studies, a diagnostic threshold of 160 HU was selected to discriminate between patients with low and normal BMD (mean sensitivity: 73.9%, mean specificity: 70.6%) (16, 17).
3.3. Statistical Analysis
Independent-samples t-test and chi-square test were performed to assess the association between the 24-hour urine biometrics, BMD, and stone size. A linear regression analysis and correlation coefficient test were used to evaluate 24-hour urine biometrics and BMD. Moreover, the normal distribution of data was evaluated using Kolmogorov-Smirnov test. Skewness and kurtosis of data were also assessed. The significance of ANOVA results was determined using Scheffe’s post-hoc test. Besides, a linear regression analysis was used to evaluate the possible cofounding effects of age and gender on the main variables of the study. A significant statistical difference was defined as a two-sided P-value less than 0.05. All statistical analyses were performed in IBM SPSS version 22.
4. Results
This study was conducted on 148 patients with urinary stones, including 85 males (57.4%) and 63 females (42.6%). Eighty-three patients were assigned to the normal BMD group (> 160 HU) and 65 patients to the low BMD group (< 160 HU). The mean age of the participants was 48.80 ± 14.11 years. Table 1 presents the basic biometric information of the patients (in two groups of low and normal BMD), including the mean stone size, mean BMI, and serum biochemical composition. Our analysis confirmed the normal distribution of data (P < 0.05). The skewness and kurtosis of all quantitative variables fell in the normal range of -2 to +2. While a direct association was observed between BMI and bone mineral attenuation (P < 0.05), no significant relationship was found between BMD and the serum biochemical composition.
| Characteristics | Low BMD (HU < 160)a (n = 65) | Normal BMD (HU > 160)a (n = 83) | P-value |
|---|---|---|---|
| Mean stone size | 37.62 ± 15.68 | 24.65 ± 9.2 | 0.03 |
| Mean age | 56.54 ± 9.89 | 43.6 ± 14.21 | 0.04 |
| Gender (M/F) | 32/33 | 53/30 | 0.07 |
| Mean BMI | 23.85 ± 4.59 | 27.97 ± 4.6 | 0.03 |
| Serum calcium | 8.34 ± 1.31 | 8.22 ± 1.07 | 0.14 |
| Serum phosphorous | 3.14 ± 1.09 | 3.26 ± 0.89 | 0.21 |
| Serum PTH level | 35.92 ± 13.62 | 33.76 ± 14.28 | 0.19 |
| Serum vitamin D level | 11.03 ± 6.91 | 28.58 ± 12.47 | 0.06 |
Abbreviations: BMI, body mass index; HU, Hounsfield unit; PTH, Parathyroid hormone; BMD, bone mineral density.
aAssessment of bone mineral attenuation was performed by designating an oval ROI with an approximate area of 2 cm2 at the L1 vertebra over the centermost area of the trabecular bone.
Table 2 presents the association between 24-hour urinalysis biometrics and BMD, with a diagnostic cut-off value of 160 HU as the discriminatory threshold. The three main urinary biomarkers (calcium, oxalate, and citrate) were compared between the two groups regarding BMD (19). A significant inverse correlation was observed when comparing urinary calcium and urinary oxalate excretions between subjects with low and normal BMD. Conversely, urinary citrate was directly correlated with bone mineral attenuation (P < 0.05 for all correlations).
| 24-Hour urinalysis | Low BMD (HU < 160) (n = 65) | Normal BMD (HU > 160) (n = 83) | P-value |
|---|---|---|---|
| Calcium | 307.95 ± 134.26 | 203.65 ± 99.01 | 0.02 |
| Oxalate | 53.22 ± 21.32 | 36.16 ± 11.77 | 0.05 |
| Citrate | 264.27 ± 187.02 | 441.24 ± 195.45 | 0.01 |
Abbreviations: HU, Hounsfield unit; BMD, bone mineral density.
Moreover, the urinary stone size data were extracted from the hospital database and defined as previously stated. Stone size was divided into three subdomains of < 20 mm, 20 - 30 mm, and > 30 mm, as established in the literature (20-22). Also, 24-hour urine biometric features were distributed and statistically evaluated across the three stone size subdomains (Table 3). Comparison of urinary biometrics among different groups based on stone size by ANOVA test showed a significant direct association between 24-hour urinary calcium and oxalate excretions and stone size, while the amount of urinary citrate excretion was inversely correlated with the urinary stone diameter (P < 0.05). The significance of the results was investigated using Scheffe’s post-hoc test (in the calcium subdomain, < 20 mm and 20-30 mm: post-hoc P-value = 0.01, < 20 mm and > 30 mm: P = 0.01, and 20 - 30 mm and > 30 mm: P = 0.02; in the oxalate subdomain, < 20 mm and 20 - 30 mm: P = 0.03, < 20 mm and > 30 mm: P = 0.03, and 20 - 30 mm and > 30 mm: P = 0.04; and in the citrate subdomain, < 20 mm and 20 - 30 mm: P = 0.01, < 20 mm and > 30 mm: P = 0.02, and 20 - 30 mm and > 30 mm: P = 0.02).
| 24-Hour urinalysis | Mean stone size groups, mm | P-value | ||
|---|---|---|---|---|
| < 20 (n = 39) | 20 - 30 (n = 52) | > 30 (n = 57) | ||
| Calcium | 138.95 ± 43.0 | 201.06 ± 94.92 | 339.56 ± 109.54 | 0.01 |
| Oxalate | 26.9 ± 4.40 | 34.91 ± 5.07 | 58.36 ± 18.04 | 0.03 |
| Citrate | 633.95 ± 108.197 | 448.44 ± 103.95 | 163.67 ± 57.45 | 0.01 |
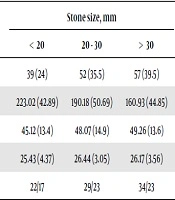
Table 4 demonstrates the relationship between urinary stone size and basic variables, including BMI, age, gender, and bone mineral attenuation for each stone size subdomain. The results of ANOVA test showed that BMD was inversely correlated with the mean urinary stone size. Patients with larger stones had a lower BMD (P < 0.05). In contrast, no significant association was found between the stone size and other parameters. The significance of data was confirmed by Scheffe’s post-hoc test (HU subdomains; < 20 mm and 20 - 30 mm: P = 0.02; < 20 mm and > 30 mm: P = 0.03; and 20 - 30 mm and > 30 mm: P = 0.03). The linear regression analysis showed that gender and age had no confounding effects on the data (P > 0.05). Also, a linear regression analysis and correlation coefficient test were used to examine the predictive role of urinary biometrics in BMD. However, none of these variables could predict BMD (P > 0.05).
| Stone size, mm | P-value | |||
|---|---|---|---|---|
| < 20 | 20 - 30 | > 30 | ||
| N = 148 | 39 (24) | 52 (35.5) | 57 (39.5) | |
| Mean bone mineral attenuation (HU) | 223.02 (42.89) | 190.18 (50.69) | 160.93 (44.85) | 0.03 |
| Mean age, y | 45.12 (13.4) | 48.07 (14.9) | 49.26 (13.6) | 0.07 |
| Mean BMI | 25.43 (4.37) | 26.44 (3.05) | 26.17 (3.56) | 0.18 |
| Gender (M/F) | 22/17 | 29/23 | 34/23 | 0.11 |
Abbreviation: BMI, body mass index; HU, Hounsfield unit.
5. Discussion
The results of this study indicated a possible correlation between 24-hour urinalysis and BMD per abdominopelvic CT attenuation in urinary calcium stone formers. Higher urinary calcium (307.95 ± 134.26 vs. 203.65 ± 99.01) and oxalate (53.22 ± 21.32 vs. 36.16 ± 11.77) excretions were observed in the low BMD group, while hypercitraturia was more common in the normal BMD group (264.27 ± 187.02 vs. 441.24 ± 195.45). Therefore, the bone mineral net loss was directly correlated with the amount of urinary calcium and oxalate elimination, while it was inversely correlated with urinary citrate. As mentioned before, the participants in this study had calcium-predominant stones, including both calcium oxalate and calcium phosphate, with the majority having calcium oxalate stones. The basic pathogenesis of these subtypes remains similar, making deduction based on these findings feasible (5, 13).
The correlations between stone size, BMD, and urinary biometrics were also evaluated in this study. The present findings suggested a significant inverse association between BMD and the urinary stone size. Larger stones were observed in low-BMD patients, and expectedly, the urinary chemical composition in patients with larger stones was similar to that of low-BMD patients; there was a significant correlation between urinary oxalate, citrate, and calcium excretions and the mean urinary stone size (P < 0.05).
Patients with urinary stones are known to have a lower BMD than the general population; therefore, they may have a higher risk of fractures, as shown in previous studies (7, 12, 13, 23). While the etiology of this phenomenon is not clearly defined (7, 9, 24, 25), the loss of BMD may be attributed to hypercalciuria, as the most common metabolic finding in patients with urinary stones (26). Almost 80% of urinary stones contain calcium as their major component (5, 13, 27, 28). In these patients, a negative ion balance is expected to directly affect the BMD, since bones are the main calcium storage sites in the human body (29, 30).
Previous studies have demonstrated an association between chronic nephrolithiasis and reduced BMD. In this regard, Asplin et al. (31) and Pietschmann et al. (7) proposed an inverse association between urinary calcium excretion and reduced lumbar and femoral BMD, based on the gold standard DEXA imaging (7, 31). Nevertheless, the direct effects of urinary calcium excretion on BMD in urinary stone patients are subject to controversy. A study by Sakhaee et al. (9) questioned the relationship between hypercalciuria and BMD in patients with urinary stones, while Tugcu et al. (11) found decreased BMD in patients with normocalcemic stones. These discrepancies may be related to the multifactorial nature of hypercalciuria and BMD disease in nephrolithiasis or differences in sample selection, as several etiologies other than urinary calcium excretion have been proposed to contribute to bone mineral loss in case of recurrent urinary stone formation.
Previous studies have documented an inverse association between urinary sodium and spinal bone content loss (7), while further research by Sakhaee et al. (9) demonstrated a relationship between higher urinary calcium levels and lower BMD in postmenopausal women without hormone replacement therapy. While previous studies, such as the cohort conducted by Framingham et al., have established the protective effect of BMI on BMD (32), the effect of serum biochemical composition on the bone mineral content is controversial and beyond the scope of this study.
Regardless of the serological and urine chemistry results, screening for reduced BMD and the subsequent increased risk of fracture is of great importance. This study proposed non-contrast CT imaging as a standard diagnostic modality for urinary stones and a promising screening tool for these patients. While our study did not have access to the gold standard DEXA imaging for measuring BMD, the 160-HU threshold provided acceptable sensitivity and specificity in distinguishing normal from osteoporotic patients, based on previous studies (5, 16, 17)
Different cutoff values, such as 180 HU and 190 HU, have been proposed in previous studies, offering higher sensitivity for differentiating osteoporotic from osteopenic patients; however, use of such thresholds was beyond the scope of the current study (16, 17, 33). The 24-hour urinalysis has been recommended in the AUA guidelines for high-risk and chronic stone formers (18). Also, abnormalities in the urine chemical composition, such as hypercalciuria, hypocitraturia, and hyperoxaluria, are common findings in these patients, which have been recognized as risk factors for recurrent stone formation and bone mineral reduction.
Appropriate treatment regimens may reduce both kidney stone recurrence and fracture risk in patients (34, 35). However, up to 35% of patients with urinary stones have normal urine biometrics in the 24-hour analysis (19). The relationship between urine biometrics and bone mineral attenuation on CT images, as established in our study, can be useful for the mentioned cases, since the additional data provided by non-contrast CT may help clinicians in risk-assessment to decide if the patient can benefit from subsequent diagnostic or curative interventions.
Our findings suggested non-contrast abdominopelvic CT imaging as a promising and valid screening tool for examining the bone mineral content in patients with urinary stones. Categorization of patients into normal and low BMD groups based on the trabecular bone attenuation on CT images (discriminatory cut-off value of 160 HU) can help clinicians differentiate high-risk patients. Also, the association between BMD and urine chemical composition is useful in selecting patients who can benefit from further work-up, either through 24-hour urinalysis or medical management.
Moreover, high-risk patients with urinary stones have been shown to have larger stone burdens. Our findings demonstrated a similar relationship between the urinary stone diameter and 24-hour urinalysis biometrics and also between BMD and urine chemical composition. Besides, stone size was shown to be inversely correlated with BMD as an independent factor. Such an association has been partially discussed in the literature (5). In this regard, Patel et al. reported an inverse association between the stone volume and BMD in a subset analysis. Overall, it seems that stone size can help physicians identify kidney stone formers with a higher risk of fracture.
To the best of our knowledge, this is the first study evaluating the association between 24-hour urinalysis and low BMD, retrieved from the CT images of an Iranian sample population. However, some limitations must be addressed. First, although previous studies have confirmed a low BMD diagnostic threshold of 160 HU in CT images to be valid compared to DEXA, our study was only conducted based on CT results, and we did not have access to the gold standard DEXA. Also, 160 HU is the defined cut-off threshold for osteopenia; therefore, there may be a sample of patients with normal BMD, falsely included in the low BMD subgroup.
Second, lack of comprehensive patient profiles in the hospital database may partly affect the results of our analysis, as several etiologies have been proposed for BMD loss in urinary stone formers. Also, history of urinary stone symptoms and the medications used by the patients might have also affected the results (1). While we retrieved the urinary and serum biochemical data of patients from the database, their history could not be fully retrieved. Therefore, further analysis of prospectively selected groups is recommended to evaluate the risk factors for fracture risk and low BMD in patients with urinary stones.
Third, an inclusion criterion of this study was undergoing PCNL. Since patients with stones smaller than 10 - 15 mm in diameter are not candidates for this type of surgery, they were not included in our analysis. Therefore, further research may be necessary to clarify the effects of smaller urinary stones on the BMD and urinary biometrics (15).
In conclusion, our findings related to reduced BMD and urinary stone size, retrieved from non-contrast abdominal/pelvic CT images, were significantly associated with abnormal 24-hour urine biochemistry. Patients with hypercalciuria, hypocitraturia, and hyperoxaluria had a lower BMD, as well as larger urinary stones. These findings can be used as a valuable screening tool to identify urinary stone patients with a higher risk of osteoporosis and fracture and to help physicians deal with such patients properly using laboratory and medical tools.