1. Background
Hepatocellular carcinoma (HCC) is currently the fourth leading malignant tumor and the second fatal tumor in China, posing serious threats to the health and life of individuals (1). Nevertheless, approximately 70% of patients who are diagnosed with HCC are in advanced stages according to the Barcelona Clinic of Liver Cancer (BCLC) staging system, and it is not appropriate to treat them with liver transplantation, surgical resection, or local ablation treatment.
According to the available guidelines, conventional transarterial chemoembolization (cTACE) is the primary method for the treatment of surgically unresectable HCC (2-4). However, the objective response rate (ORR) of cTACE in patients with advanced liver cancer is estimated at 15 - 55%. Due to incomplete tumor necrosis following cTACE, besides the formation of tumor collateral vessels and the overexpression of vascular endothelial growth factor (VEFG), some patients exhibit cTACE resistance, making this treatment more challenging (5). Also, some studies have reported survival benefits for adjuvant TACE therapy in only some patients (6, 7).
Drug-eluting bead-TACE (DEB-TACE) has been used as an embolization system in recent years, which can not only load chemotherapy drugs and release them slowly in local regions, but also embolize tumor-supply vessels permanently (8). Drug-eluting bead transarterial chemoembolization is more efficient than cTACE and equally tolerated by HCC patients with a history of multiple cTACE treatments (7). However, there is a lack of studies on the treatment of liver cancer patients with cTACE resistance.
2. Objectives
Since 2017, DEB-TACE has been employed in our center to treat HCC patients with cTACE resistance. The present study aimed to investigate the treatment benefits and side effects of DEB-TACE in HCC patients with cTACE resistance.
3. Patients and Methods
3.1. Patients
A total of 17 HCC patients, who were cTACE resistant, were recruited from Panzhihua Central Hospital (Sichuan, China) from July 2017 to December 2019. The inclusion criteria were as follows: (1) Meeting the criteria for cTACE-resistant HCC according to the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) (2); (2) intrahepatic tumors ≤ 3 cm and expected survival time > 6 months; (3) written informed consent before DEB-TACE; (4) not receiving other anti-tumor treatments from the time of cTACE resistance until DEB-TACE treatment; (5) a Child-Pugh score < 10; and (6) complete medical records. Patients whose main portal vein was not completely blocked, as well as patients whose hepatic artery and portal vein were completely blocked, but showed formation of compensatory collateral blood vessels, were also included in this study. The exclusion criteria were as follows: (1) Patients with secondary liver cancer; and (2) patients who were lost to follow-up and had no follow-up records.
3.2. Definitions
The definition of cTACE resistance was based on the guidelines proposed by Japan Liver Cancer Research Association in 2014 (5). The presence of one of the following four conditions was regarded as cTACE resistance: (1) After two or more cTACEs, even if the chemotherapy drug regimen is changed, and/or other tumor nourishing arteries are affected, computed tomography (CT)/magnetic resonance imaging (MRI) shows residual lesions > 50%, or new lesions appear after one to three months; (2) alpha-fetoprotein (AFP) continues to increase in the short term after cTACE; (3) vessel invasion appears; and (4) distant metastasis occurs. This study was approved by the Ethics Committee of Panzhihua Central Hospital (No: 20180008). Informed consent was obtained from all the participants.
3.3. Drug Loading Methods
A microsphere syringe, containing CalliSpheres Beads (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China), was prepared (20 mL) and left for about two minutes. After the microspheres settled, the supernatant was slowly removed and discarded. Moreover, a syringe containing chemotherapy drugs was prepared by adding 3 mL of 5% glucose (10 mL) to dissolve 60 mg of epirubicin. The microspheres and chemotherapy drugs were completely mixed with a three-way stopcock. During the loading procedure, the mixture was shaken every five minutes to allow the microspheres to be fully loaded with drugs within 30 minutes. Once loaded, the DEB microspheres and a non-ionic contrast agent were completely mixed at a volume ratio of 1: 1 and used after two minutes. In this study, the particle size of drug-loaded microspheres was selected according to the tumor size and blood supply. Considering the individual differences, the particle size was inconsistent in this study. For all the patients, microspheres with a diameter of 100-300 μm were used.
3.4. Drug-Eluting Bead Transarterial Chemoembolization Treatment Procedure
All patients underwent angiography of the celiac artery and/or superior mesenteric artery to determine the tumor location, size, and number, tumor blood vessels, and tumor blood supply, as well as portal vein tumor thrombus and hepatic arteriovenous fistula. A 2.7F Progreat microcatheter (Terumo Corporation, Japan) was used for arteriography of tumor blood supply. Next, the DEB microspheres were extracted using a 1-mL syringe, and they were slowly injected into the tumor in vivo. After the blood flow of the tumor target blood vessel decelerated significantly (3 - 4 cardiac cycles; the microspheres did not empty in the blood vessel), subsequent angiography was performed to identify the extent of vascular occlusion. Polyethylene microspheres (Jiangsu Hengrui Pharmaceuticals Co., Ltd., Jiangsu, China) were applied for embolization as appropriate.
A microcatheter was used for superselecting the tumor supply artery. After confirming the correct position of this artery, the microcatheter was used at a slow and stable flow rate (1 mL/min), according to factors, such as the tumor location, patient’s age, and patient’s pain threshold. A suspension of drug-loaded microspheres, chemotherapeutics, and contrast agents was intermittently and slowly injected into the tumor supply artery. If there was an obvious arteriovenous shunt, suitable embolic materials, such as gelatin sponge or polyvinyl alcohol (PVA) particles, would be first used, the shunt would be blocked, and then, the drug-loaded microspheres would be injected. For the bolus injection of microspheres, they were kept evenly distributed in the syringe to avoid precipitation; a 1-mL syringe was used each time, based on the pulse injection method while injecting and shaking the syringe.
The tumor was embolized branch by branch, depending on the blood supply of the tumor, and the flow rate of the chemotherapeutic drug and the contrast medium suspension was monitored; the bolus injection was terminated when the embolization endpoint was reached; the contrast agent was not drained for three to four cardiac cycles, which could be regarded as complete embolism rather than blood flow stagnation. After pausing for five minutes, angiography was performed again. If tumor staining persisted, embolization continued until reaching the end point of embolization (disappearance of tumor staining).
3.5. Assessment of Clinical Efficacy
Routine blood and liver function biochemical tests were performed one week after DEB-TACE. The follow-up routine blood tests, liver function tests, tumor markers, and enhanced CT or MR imaging of the upper abdomen were performed and evaluated again at six and 12 weeks after treatment, based on the modified response evaluation criteria in solid tumors (mRECIST) (9, 10). The intrahepatic tumor lesions were also evaluated by two experienced radiologists (with more than five years of work experience) in cooperation with our department.
Complete remission (CR) was defined as disappearance of any target tumor enhancement; partial remission (PR) was defined as a reduction in the total diameter of target tumor by ≥ 30%; stable disease (SD) was defined as a reduction in the overall diameter of target tumor by less than 30% or an increase of < 20%; and progression of disease (PD) was regarded as an increase in the diameter of target tumor by ≥ 20% or emergence of new lesions. Moreover, the objective remission rate (ORR) refers to the percentage of CR + PR patients in the total population, while the disease control rate (DCR) refers to the percentage of CR + PR + SD patients in the total population.
Additionally, the AFP levels were recorded before treatment and six and 12 weeks post-treatment. Patients who were CR after the first DEB-TACE were included in the follow-up. For patients who were PR, SD, or PD after the first procedure, if necessary, DEB-TACE was repeated based on the assessment of liver function; they were then included in the follow-up study. On the other hand, patients who had undergone treatment, but still had PD, were eliminated, and sorafenib therapy or palliative treatment was recommended instead of DEB-TACE.
3.6. Assessment of Clinical Safety
The common terminology criteria for adverse events (CTCAE V5.0) (11) were applied to evaluate the adverse reactions of patients after DEB-TACE, including fever, nausea, vomiting, abdominal pain, and abdominal distension. The following parameters were recorded in the first and sixth weeks after DEB-TACE: (1) Liver enzymes, including serum albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TB) levels; (2) routine blood tests, including white blood cell (WBC), red blood cell (RBC), and platelet (PLT) counts; and (3) AFP levels.
3.7. Prevention and Treatment of Side Effects and Complications of Drug-Eluting Bead Transarterial Chemoembolization
The adverse reactions and complications of DEB-TACE need to be prevented and managed. The most common adverse reaction to DEB-TACE is post-embolization syndrome, which mainly manifests as liver pain, fever, nausea, and vomiting. Other adverse reactions include liver damage and bone marrow suppression, caused by chemotherapy drugs. In this study, if a patient showed an adverse reaction, it would be managed as follows:
Pain: The patient's pain level was assessed according to the visual analogue scale (VAS). If a patient experienced pain, it would be treated with 100 mg of tramadol by an intramuscular injection and 40 mg of parecoxib by an intravenous bolus injection.
Fever: If a patient had a low-grade fever, physical cooling was applied. If a patient had a moderate to high fever, non-steroidal anti-inflammatory drugs, such as indomethacin suppository (50 mg), could be used orally. If the body temperature exceeded 39°C, co-infection and antibiotic treatment should be considered.
Nausea and vomiting: If a patient experienced nausea, vomiting, or other discomforts, gastrointestinal and antiemetic medications, such as ondansetron hydrochloride (8 mg) or tropisetron hydrochloride (5 mg), could be administered via intramuscular injection for treatment.
Liver damage: If a patient showed liver damage, s/he was routinely administered liver medications to reduce transaminase levels for treatment.
Bone marrow suppression: If a patient exhibited bone marrow suppression, it would be treated symptomatically according to the degree of bone marrow suppression.
3.8. Statistical Analysis
SPSS version 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) was used for statistical analysis. The normal distribution of data was evaluated using Shapiro-Wilk test. All continuous variables are expressed as mean ± standard deviation (SD). Statistical differences (liver enzymes, routine blood tests, and AFP levels) between the groups were assessed using paired t-test for continuous variables. All statistical analyses were based on two-tailed hypothesis tests at a significance level of P ≤ 0.05.
4. Results
4.1. Baseline Clinical Characteristics
In this retrospective study, 17 patients with cTACE resistance (14 males and 3 females; mean age, 55.7 ± 75 years; mean tumor diameter, 7.2 ± 2.3 cm) were evaluated from July 2017 to December 2019. Fourteen patients were evaluated by CT scan, and three patients were evaluated by MRI. None of the patients in this study were treated with sorafenib therapy. The baseline clinical characteristics of the patients are presented in Table 1.
| Variables | Values |
|---|---|
| Sex | |
| Male | 14 (82.4) |
| Female | 3 (17.6) |
| Age (y) | 55.7 ± 5.0 |
| Number of cTACE procedures before DEB-TACE | 3 ± 1 |
| Etiology | |
| HBV | 15 (88.2) |
| Alcohol intake | 1 (5.9) |
| Others | 1 (5.9) |
| Liver enzymes and AFP levels | |
| AFP (μg/L) | 425.5 ± 390.2 |
| TB (μmol/L) | 20.4 ± 8.0 |
| ALB (g/L) | 36.5 ± 4.8 |
| AST (U/L) | 65.7 ± 23.1 |
| ALT (U/L) | 70.0 ± 33.7 |
| Characteristics of tumors | |
| Diameter (cm) | 7.2 ± 2.3 |
| Vascular invasion | 2 (11.8) |
| Multiple nodules | 7 (41.2) |
| Large lump | 8 (47.0) |
| BCLC stage | |
| A | 14 (82.4) |
| B | 3 (17.6) |
| ECOG PS | |
| 0 | 0 |
| 1 | 15 (88.2) |
| 2 | 2 (11.8) |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
Abbreviations: cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting bead transarterial chemoembolization; AFP, alpha-fetoprotein; TB, total bilirubin; ALB, serum albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic of Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HBV, hepatitis B virus.
a Values are expressed as No. (%) or mean ± standard deviation.
4.2. Treatment Effects
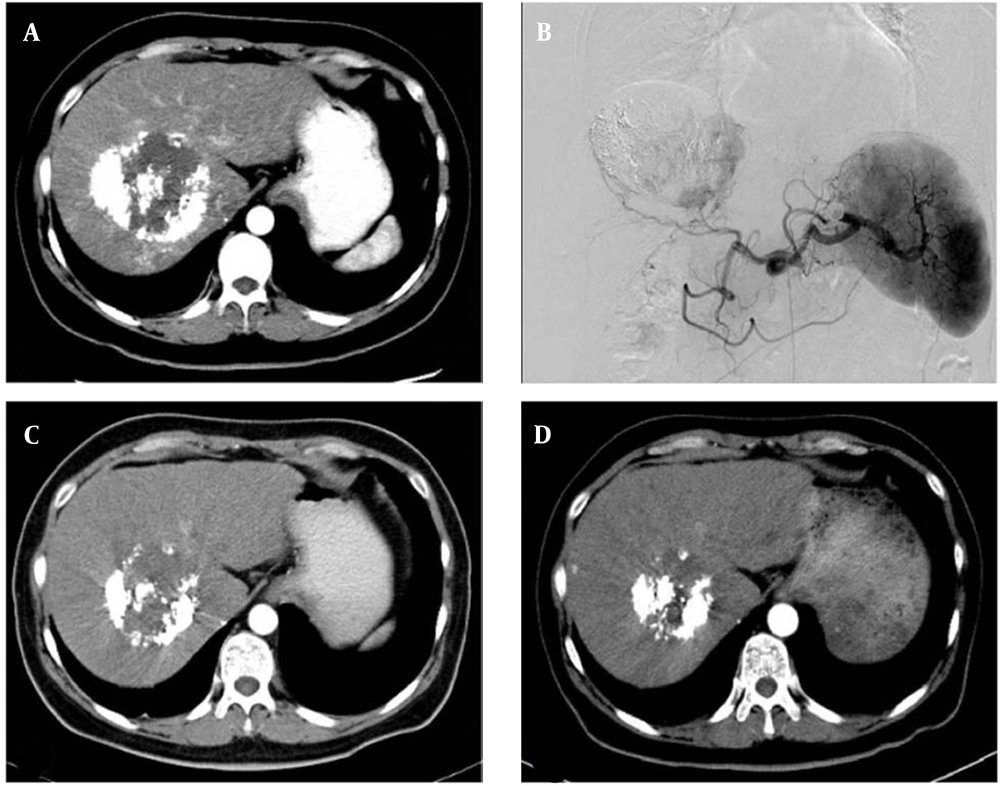
The technical success rate of DBE-TACE was 100% in 17 patients. A total of 28 target lesions were treated in this study. According to the mRECIST standards, CR PR, SD, and PD were reported in 0, 6, 8, and 3 patients at six weeks post-treatment and in 1, 8, 5, and 3 patients at 12 weeks post-treatment, respectively. The ORR and DCR were estimated at 35.29% and 82.35% in the sixth week and 52.94% and 82.35% in the 12th week, respectively (Figure 1A - D). The local tumor response rates are summarized in Table 2.
The therapeutic effect of drug-eluting bead transarterial chemoembolization (DBE-TACE) procedure. A, A 48-year-old hepatocellular carcinoma female patient underwent conventional transarterial chemoembolization twice. Lipiodol accumulation can be seen in the tumor, and a patchy enhancement can be seen in the central and marginal areas of the tumor. B, Digital subtraction angiography confirms the CT signs in A. C, Six weeks after DBE-TACE, CT shows that the tumor size is significantly reduced, and only few light enhancement signs can be seen. D, Ten weeks after DBE-TACE, CT shows that the tumor size is further reduced, and there are no definite signs of enhancement in or around the tumor.
| Variables | Week 6 | Week 12 | ||
|---|---|---|---|---|
| Patients | Lesions | Patients | Lesions | |
| CR | 0 | 4 (14.29) | 1 (5.88) | 6 (21.43) |
| PR | 6 (35.29) | 10 (35.71) | 8 (47.06) | 12 (42.86) |
| SD | 8 (47.06) | 9 (32.14) | 5 (29.41) | 6 (21.43) |
| PD | 3 (17.65) | 5 (17.86) | 3 (17.65) | 4 (14.29) |
| ORR (%) | 35.29 | / | 52.94 | / |
| DCR (%) | 82.35 | / | 82.35 | / |
Abbreviations: CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; ORR, objective remission rate; DCR, disease control rate
a Values are expressed as No. (%) unless otherwise indicated.
In the first week after DBE-TACE, the levels of ALT, AST, TB, and WBC significantly increased, while ALB, RBC, and PLT did not change significantly. The levels of ALT (P = 0.01), AST (P = 0.02), TB (P = 0.02), and WBC (P = 0.001) were significantly higher in the first week after DBE-TACE compared to the pre-treatment stage, while there were no significant differences in the levels of ALB, RBC, PLT, or AFP. Also, six weeks after DBE-TACE, there were no significant differences in liver enzymes, routine blood parameters, or AFP levels compared to the pre-treatment stage (Table 3).
| Variables | Before DEB-TACE | Week 1 after DEB-TACE | P-Value b | Week 6 after DEB-TACE | P-Value c |
|---|---|---|---|---|---|
| ALT (U/L) | 70.0 ± 33.7 | 107.5 ± 44.4 | 0.01 | 62.8 ± 24.6 | 0.48 |
| AST (U/L) | 65.7 ± 23.1 | 93.0 ± 37.2 | 0.02 | 63.0 ± 21.1 | 0.72 |
| TB (µmol/L) | 20.4 ± 8.0 | 27.9 ± 10.3 | 0.02 | 18.5 ± 7.0 | 0.48 |
| ALB (g/L) | 36.5 ± 4.8 | 35.6 ± 5.4 | 0.61 | 37.0 ± 3.2 | 0.77 |
| WBC (×109/L) | 7.1 ± 1.5 | 10.3 ± 3.0 | 0.00 | 6.6 ± 1.5 | 0.38 |
| RBC (×1012/L) | 4.5 ± 0.4 | 4.6 ± 0.5 | 0.24 | 4.4 ± 0.3 | 0.15 |
| PLT (×109/L) | 117.0 ± 51.0 | 131.0 ± 38.0 | 0.10 | 143 ± 76.8 | 0.09 |
| AFP (µg/L) | 425.5 ± 390.2 | 375.5 ± 341.8 | 0.56 | 303.0 ± 246.7 | 0.44 |
Abbreviations: DEB-TACE, drug-eluting bead transarterial chemoembolization; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; ALB, serum albumin; WBC, white blood cells; RBC, red blood cells; PLT, platelets; AFP, alpha-fetoprotein.
a Values are expressed as mean ± standard deviation.
b Comparison of pre-DEB-TACE phase with the one-week follow-up after DEB-TACE using paired t-test
c Comparison of pre-DEB-TACE phase with the six-week follow-up after DEB-TACE using paired t-test
4.3. Assessment of Safety
In the first week after DEB-TACE, the most common complication of DEB-TACE was post-embolization syndrome, including nausea, vomiting, abdominal distension, abdominal pain, and fever, which occurred in all 17 patients. Since the symptoms were all mild, after symptomatic treatment, all the symptoms were relieved within one week. No severe complications, such as liver failure, massive pleural or abdominal effusion, cholecystitis, or gastrointestinal bleeding, occurred. The classification of adverse reactions is summarized in Table 4.
| Variables | Classification | |||||
|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | V | |
| Nausea | 6 (35.3) | 10 (58.8) | 1 (5.9) | 0 | 0 | 0 |
| Vomiting | 7 (41.2) | 8 (47.1) | 2 (11.8) | 0 | 0 | 0 |
| Abdominal distension | 13 (76.5) | 4 (23.5) | 0 | 0 | 0 | 0 |
| Abdominal pain | 8 (47.1) | 9 (52.9) | 0 | 0 | 0 | 0 |
| Fever | 11 (64.7) | 5 (29.4) | 1 (5.9) | 0 | 0 | 0 |
a Values are expressed as No. (%).
5. Discussion
The DEB microspheres are characterized by a uniform particle diameter and a smooth surface and exhibit biocompatibility, morphological plasticity, and high vascular compliance (8). Drugs are commonly loaded by ion exchange; they can be loaded with anthracyclines, such as epirubicin and pirarubicin, or DNA topoisomerase inhibitors, such as irinotecan (12). Drug-eluting bead has the advantage of permanent embolization of tumor blood supply arteries, which can load chemotherapy drugs and release them slowly in local regions (13). After entering the tumor blood vessels, the external environment of DEB changes, and the loaded drugs exchange with other ions in the blood to break away from DEB. Chemotherapeutic drugs are slowly released into the tumor at a continuously high concentration, which is synergistically inhibited at the beginning and at an effective concentration, and tumor cells are exposed to greater chemotherapeutic effects compared to pure perfusion (14, 15).
In a study by Namur et al. (12), nearly 40% of chemotherapeutic drugs loaded by DEB were released within the first month, and 90% of chemotherapeutic drugs were released within three months. Therefore, DEB-TACE showed dual effects of local continuous chemotherapy and permanent embolization of tumor blood vessels. This method is currently recognized as one of the most effective methods for the non-surgical treatment of advanced HCC.
Additionally, in a study by Lee et al. (16), the CR and ORR of patients who underwent DEB-TACE were 40.1% and 91.4% within one month after treatment and 43.0% and 55.4% within six months after DEB-TACE, respectively. Moreover, Cun et al. (17) reported that the CR, PR, SD, PD, ORR, and DCR of patients were 33.3%, 43.8%, 16.0%, 6.0%, and 74.0% at three months after DEB-TACE, respectively. In this study, DEB-TACE was used to treat HCC patients with cTACE resistance. The ORR and DCR were estimated at 35.29% and 82.35% at six weeks after DEB-TACE and 52.94% and 82.35% at 12 weeks after therapy, respectively. However, the overall patient-related treatment benefits were lower than other reports, which might be due to resistance following multiple cTACE treatments, liver function, tumor volume, and degree of liver cirrhosis in patients recruited in this study.
The cTACE is characterized by limited drug concentrations and limited time for maintaining chemotherapeutic drugs in blood vessels supplying the tumors; therefore, it is difficult to completely embolize the tumor blood vessels (18). Due to the incomplete tumor necrosis, massive secretion of growth factors (caused by tumor hypoxia), formation of collateral blood supply vessels, tumor drug resistance and the low efficacy of cTACE, which has been reported in some patients, lead to cTACE resistance, which manifests as intra- and extra-hepatic metastasis, vascular invasion, a continuous increase in the level of tumor markers, and rapid tumor progression after multiple cTACE treatments (19).
In this study, DEB-TACE was used to treat patients with cTACE resistance. The results indicated the therapeutic effects of this treatment, which might be related to the ability of DEB to embolize the peripheral blood vessels of tumors in a sustainable manner and to release drugs in tumor cells at a slow rate and a high concentration. According to previous research, if a patient has increased levels of AFP or shows tumor recurrence after the first DEB-TACE, they should be removed from the study and receive sorafenib therapy or other conservative treatments (20). Sorafenib therapy has led to breakthroughs in the treatment of advanced HCC (21). Studies have shown that sorafenib, combined with TACE, can be advantageous for patients with advanced HCC (22). However, sorafenib therapy is not a popular method due to its side effects, low tolerance, high price, and low effectiveness.
Hepatic arterial infusion chemotherapy (HAIC), as one of the standard treatments for advanced HCC, has particular effects on patients who cannot undergo embolization (23). Zhang et al. (14) explored the use of low-dose chemotherapeutics in hepatic arterial chemoembolization and reported their positive effects in elderly patients with low chemotherapy tolerance. Additionally, in 2000, Hanahan et al. (24) proposed the concept of metronomic therapy, which refers to a continuous low-dose administration of chemotherapy drugs to inhibit tumor angiogenesis (also known as anti-neovascular chemotherapy). Recently, increasing attention has been paid to these anti-tumor treatment models. Further research is needed to determine how to facilitate a continuous, effective, and controlled entry of small drug concentrations into tumors, how to reduce the side effects of poor liver function, and how to reduce the side effects of systemic chemotherapy drugs while performing DEB-TACE. It is worth mentioning that this retrospective study only included patients treated with DEB-TACE, which was effective in tumor control.
The rate of DEB-TACE complications, including embolic syndrome, abnormal liver function, biliary tract injury, pleural effusion, cholecystitis or gallbladder perforation, gastrointestinal bleeding, and liver abscess, is estimated at 4.2% to 11.4% (25). This study found that the side effects of DEB-TACE and cTACE were basically similar, while the symptoms were mild and alleviated after a symptomatic supportive treatment. None of the patients experienced any severe side effects, such as liver abscess, liver failure, or bile leakage. Overall, DEB-TACE seems to have theoretical and practical advantages in reducing adverse reactions in patients with advanced HCC, especially HCC patients with cTACE resistance (26).
Since DEB causes considerable blood stagnation in the vascular bed, it can lead to thrombus in the tumor location, which can lead to a lack of perfusion in the follow-up imaging. Therefore, the imaging finding can be interpreted as CR, which is not related to tumor disappearance, but rather a lack of perfusion at the tumor site, associated with a lack of enhancement. Consequently, it is important to determine whether the tumor is progressing by puncturing the liver in suspected cases.
There are several limitations in this study. First, the efficacy and safety of DEB-TACE cannot be accurately determined due to the short-term follow-up. Second, this study was a retrospective and single-center study with a small sample size. Therefore, to approve the present findings, further multicenter randomized clinical trials with a larger sample size and higher quality are needed.
5.1. Conclusions
In conclusion, in this study, DEB-TACE was used to treat HCC patients with cTACE resistance, and its short-term efficacy and adverse reactions were retrospectively evaluated. According to the HCC treatment response index of cTACE resistance, DEB-TACE has more benefits and fewer side effects compared to cTACE according to the latest guidelines; therefore, it can be promoted in the treatment of HCC patients with cTACE resistance (27).