1. Background
The number of patients with thyroid nodules has increased due to the widespread use of ultrasonography (US). While most of these nodules are benign, nearly 10% of them may be malignant (1). An accurate radiological diagnosis prevents unnecessary surgical procedures and contributes to the prolongation of life expectancy in patients with malignant lesions by leading to an appropriate treatment (2). It seems that establishment of standard guidelines can help increase communication between radiologists and clinicians.
The first Thyroid Imaging-Reporting and Data System (TI-RADS) was proposed by Horvath et al. (3). The thyroid US risk stratification has been integrated into the guidelines published by the American Thyroid Association (ATA) and the British Thyroid Association (BTA) in recent years. It has been emphasized that US findings are more important than an increased thyroid size when deciding on a biopsy (4, 5). The malignancy risk can be calculated by scoring the US findings based on TI-RADS, recommended by the American College of Radiology (ACR) (6). There are also European (EU) TI-RADS and the Society of Radiology in Ultrasound (SRU) TI-RADS guidelines (7, 8).
2. Objectives
This study aimed to retrospectively analyze the US-guided fine needle aspiration biopsy (US-FNAB) findings of the patients, to re-evaluate the findings based on Kwak TI-RADS classification (as a practical system for use in routine practice), and to compare the results with those in the literature.
3. Patients and Methods
3.1. Patient Selection
This cross-sectional study retrospectively evaluated the radiological and pathological data of 776 consecutive patients, who underwent US-FNAB in our interventional radiology clinic after presenting to the departments of general surgery, surgical oncology, otolaryngology, endocrinology, and internal medicine between March 2018 and February 2020, according to the hospital records.
The US-FNAB was performed on a single nodule. For multiple nodules, it was carried out on the dominant nodule and/or the nodule suspicious of malignancy according to Kwak TI-RADS criteria by an experienced interventional radiologist (M.C.). During and after the procedure, the patients’ data and characteristics of the nodules were recorded. The study group included patients with Kwak TI-RADS 3, 4, and 5 lesions and Bethesda II (benign) and/or Bethesda VI (malignant) cytological findings, whose biopsy samples were obtained by the same interventional radiologist (M.C.), using a similar method.
Patients who met the following criteria were excluded from the study: (1) Kwak TI-RADS 2 (purely cystic) lesions; (2) non-diagnostic cytology; (3) Bethesda III or IV cytological results; and (4) discordant cytological and histopathological diagnoses. Besides, repeat procedures and patients with insufficient data were not included in the study. A total of 641 patients, who met the inclusion criteria, were included in the study, while 38 patients with non-diagnostic cytology, 80 patients with Bethesda III/IV lesions, 16 patients with Bethesda IV lesions, and one patient with discordant pre- and postoperative pathological diagnoses were excluded (Figure 1).
3.2. US Examinations
The US and US-FNAB procedures were performed using a Toshiba Aplio 300 ultrasonography system (Toshiba Medical System, Tokyo, Japan) and a 13-MHz linear-array transducer. After local cleaning, aspiration was performed through a back-and-forth maneuver inside the nodule, using a 21-gauge, green-tipped, 10-cc injector, guided by US. Aspiration of each nodule was performed three times.
3.3. Radiological Characterization of Nodules
The following characteristics were determined for each nodule: size, composition, echogenicity, borders, shape, and presence of calcifications. Size was described as the longest diameter of the nodule. Nevertheless, the association between changes in the nodule size during the follow-up and malignancy was not investigated. Regarding composition, the cystic and solid areas of the nodule were examined. Because patients with purely cystic lesions (Kwak TI-RADS 2) were not included in the study, the nodules were classified as solid or mixed (i.e., solid cystic). Also, to evaluate echogenicity, the nodule was compared with the adjacent thyroid parenchyma and classified as isoechoic or hypoechoic.
Echogenicity was evaluated in the solid part of mixed nodules. Nodules were also classified as well circumscribed, microlobulated, or poorly circumscribed with irregular margins. The nodule shape was classified into taller-than-wide or wider-than-tall, based on the length of the vertical and horizontal axes. In terms of calcification, the nodules were divided into three groups: without calcification, with microcalcification < 1 mm appearing as punctate and hyperechogenic foci, and with macrocalcification appearing as hyperechogenic foci > 1 mm.
All nodules were evaluated according to the TI-RADS system, proposed by Kwak (9). This system recommends differentiating nodules according to their suspicious characteristics, including the presence of a solid component, hypoechogenicity, microlobulated or irregular margins, microcalcifications, and a taller-than-wide shape. While a normal thyroid gland without nodules is classified as Kwak TI-RADS 1, purely cystic nodules, spongiform nodules, isolated macrocalcifications, and typical subacute thyroiditis are classified as Kwak-TI-RADS 2 and considered as benign nodules (9, 10). Because Kwak TI-RADS 2 nodules are considered benign, they were not included in the present study.
Moreover, TI-RADS 3 suggests unsuspicious/mildly suspicious nodules; TI-RADS 4a suggests that the nodule only has one suspicious feature; TI-RADS 4b suggests that the nodule has two suspicious features; TI-RADS 4c suggests that the nodule has three or four suspicious features; and finally, TI-RADS 5 suggests that the nodule has five highly suspicious characteristics of malignancy (Figures 2 and 3).
The US scans demonstrate A, A Kwak Thyroid Imaging-Reporting and Data System 2 (TI-RADS 2) lesion, compatible with typical subacute thyroiditis without nodule formation; B, A TI-RADS 3, isoechoic, well-circumscribed, mixed nodule with a wider-than-tall shape without any calcifications (this nodule was later confirmed as benign by cytological evaluation); and C, A well-circumscribed, wider-than-tall TI-RADS 4a nodule with a solid component and isohyperechoic features. While this nodule only had one suspicious feature for malignancy according to Kwak system, the cytological evaluation revealed its benign nature.
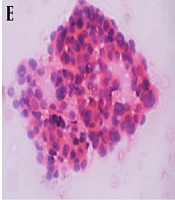
A-C, A Thyroid Imaging-Reporting and Data System (TI-RADS) 4b, isoechoic, well-circumscribed, wider than-tall nodule with a solid component and microcalcifications and two suspicious US features according to Kwak system. B-C, Cytology indicates a malignancy. Malignant fine needle aspiration biopsy (FNAB) is consistent with papillary thyroid carcinoma. Nuclear crowding and enlargement are prominent (note the nuclear grooves and nuclear contour irregularities). B, PAP staining, × 400 magnification. C, Hematoxylin & eosin (H&E) staining (cell blocks), × 200 magnification. D-F, A TI-RADS 4c nodule with a solid component, lobulated contours, and a wider than-tall shape. The nodule is hypoechoic without calcifications. This nodule, with three suspicious US features according to Kwak system, was confirmed to be malignant by (E) cytology in H&E staining at × 200 magnification. Nuclear enlargement, crowding, and superposition are prominent features. F, Similar nuclear features are observed in the resected specimen (H&E staining, × 400 magnification). G-I, Another (H) cytologically (×200 magnification) and (I) histopathologically (× 40 magnification) confirmed malignant case with a hypoechoic TI-RADS 5 nodule with solid components, microcalcifications, lobulated contours, and a taller-than-wide shape, in addition to all five suspicious US features for malignancy according to Kwak system. This case was diagnosed with classic variant multifocal papillary thyroid carcinoma.
3.4. Cytopathological Evaluation
The aspirates were cytologically evaluated, and a cytopathological diagnosis was established according to the 2017 Bethesda System for Reporting Thyroid Cytopathology (TBSRTC), which includes six main diagnostic categories: non-diagnostic, benign, atypia or follicular lesion of undetermined significance, (suspicious) follicular neoplasm, suspicious for malignancy, and malignant (11).
3.5. Statistical Analysis
Statistical analysis was performed in R-Project Version 3.6.3 (12). Patients with benign or malignant thyroid nodules were compared according to sex and US characteristics using Chi-square test. Independent samples t-test was also used to compare age between patients with benign and malignant nodules. The relationship between the US features and malignancy diagnosis was evaluated by uni- and multivariate logistic regression analyses. Besides, generalized equations for prediction were used to determine independent predictors of malignancy, based on the US features by adjusting for all variables. A P value < 0.05 was considered statistically significant.
4. Results
The majority of the patients were female (n = 513; 80%). Of 599 patients with benign nodules, 482 were female, and 117 were male. Of 42 patients with malignant nodules, 31 were female, and 11 were male. Patients with malignancy were significantly older than patients with benign nodules (49.50 ± 14.35, range: 15-96 years vs. 41.07 ± 11.65, range: 21-86 years; P < 0.01). However, no significant association was found between sex and diagnosis of malignancy (P = 0.416) (Table 1).
| Benign (n = 599) | Malignant (n = 42) | Total (n = 641) | P value | |
|---|---|---|---|---|
| Age (years) | 41.07 ± 11.65 (21-86) | 49.50 ± 14.35 (15-96) | 44.09 ± 13.72 (15-96) | < 0.01 |
| Sex | 0.416 | |||
| Female, No. (%) | 482 (80.4) | 31 (73.8) | 513 (80) | |
| Male, No. (%) | 117 (19.6) | 11 (16.2) | 128 (20) |
aValues are expressed as mean ± SD or No. (%).
All malignancy diagnoses were surgically confirmed. Of 599 benign patients, 75 underwent surgery, and their diagnosis was confirmed surgically (Table 2).
| Malignant (n = 42) | Number of patients (%) |
|---|---|
| Papillary carcinoma | 25 (59.5) |
| Follicular variant of papillary carcinoma | 16 (38) |
| Follicular carcinoma | 1 (2.3) |
| Benign (n = 75) | |
| Adenomatous hyperplasia | 61 (81.3) |
| Lymphocytic thyroiditis | 9 (12) |
| Follicular adenoma | 4 (5.3) |
| Hurthle cell adenoma | 1 (1.3) |
aValues are expressed as No. (%).
Of 268 patients classified as TI-RADS 3, only 0.7% (n = 2) were malignant. Of 222 patients classified as TI-RADS 4a, 1.4% (n = 3) were malignant. The frequency of malignancy increased with the TI-RADS category, as the percentage of malignancy in TI-RADS 4b, TI-RADS 4c, and TI-RADS 5 cases was 5.9%, 46.7%, and 89.5%, respectively (Table 3).
| Number of benign nodules (%) | Number of malignant nodules (%) | |
|---|---|---|
| TI-RADS 3 | 266 (99.3) | 2 (0.7) |
| TI-RADS 4a | 219 (98.6) | 3 (1.4) |
| TI-RADS 4b | 96 (94.1) | 6 (5.9) |
| TI-RADS 4c | 16 (53.3) | 14 (46.7) |
| TI-RADS 5 | 2 (10.5) | 17 (89.5) |
Abbreviation: TI-RADS, thyroid imaging-reporting and data system.
aValues are expressed as No. (%).
Table 4 summarizes the results of uni- and multivariate logistic regression analyses; the adjusted R2 value for the model was 0.519. The shape, composition, echogenicity, margin characteristics, and the presence of calcification were significantly associated with malignancy in the univariate logistic regression analysis (P < 0.05), as solid, hypoechoic, microlobulated, irregular, calcified, and taller-than-wide nodules were significantly more likely to be malignant (see the odds ratios [ORs] in Table 4). On the other hand, only hypoechogenicity (OR: 1.38, 95% CI: 1.39-10.86, P = 0.01) and a taller-than-wide shape (OR: 1.58, 95% CI: 1.71-20.08, P = 0.005) were significantly associated with malignancy in the multivariate analyses (Table 4).
| Parameters | No. of benign nodules (n = 599) | No. of malignant nodules (n = 42) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| P value b | OR | 95% CI | OR | 95% CI | P value b | |||
| Composition | < 0.001 | |||||||
| Solid (n = 343) | 304 | 39 | 9.429 | 3.328-26.716 | 1.831 | 0.555-6.046 | 0.321 | |
| Mixed (n = 298) | 295 | 3 | 1 | 1 | ||||
| Echogenicity | < 0.001 | |||||||
| Isoechogenicity (n = 547) | 504 | 7 | 1 | 1 | ||||
| Hypoechogenicity (n = 130) | 95 | 35 | 23.164 | 10.421-51.489 | 3.89 | 1.393-10.862 | 0.01 | |
| Margins | < 0.001 | |||||||
| Well circumscribed (n = 562) | 551 | 11 | 1 | 1 | ||||
| Microlobulated (n = 64) | 48 | 16 | 15.278 | 6.834-34.155 | 2.639 | 0.880- 7.909 | 0.083 | |
| Irregular (n = 5) | 1 | 14 | 1949771 | 0, ∞ | 92949 | 0, ∞ | 0.985 | |
| Calcifications | < 0.001 | |||||||
| Microcalcifications (n = 42) | 20 | 22 | 32.421 | 15.181-69.240 | 2.346 | 0.707-7.787 | 0.164 | |
| Macrocalcifications (n = 20) | 18 | 2 | 3,275 | 0.790-15.135 | 2.798 | 0.471-16.611 | 0.257 | |
| No calcification (n = 579) | 561 | 18 | 1 | 1 | ||||
| Shape | < 0.001 | |||||||
| Wider than tall (n = 589) | 576 | 13 | 1 | 1 | ||||
| Taller than wide (n = 52) | 23 | 29 | 51.786 | 24.172-110.947 | 5.87 | 1.716-20.081 | 0.005 | |
Abbreviation: CI, Confidence interval.
a McFadden's R2: 0.519.
b Logistic regression analysis.
5. Discussion
Thyroid nodules are being increasingly recognized due to easier access to imaging techniques, such as US (13, 14). Thyroid nodules are detected in 20-76% of healthy individuals (4, 15), and they are five times more common in women than in men (16); however, most of these nodules are benign. In the present study, although the female-to-male ratio was 4:1, no significant association was found between sex and malignancy, similar to the findings reported by Kwak et al. (9). On the other hand, our malignant cases were significantly older than benign cases, which is inconsistent with the literature (9, 10) and may reflect population differences.
There are several guidelines on the risk classification of thyroid nodules (9, 14, 15, 17, 18), providing useful information for the US examination in FNAB procedures. In this study, the TI-RADS classification proposed by Kwak (9) was used, because it is a practical and highly sensitive system with a similar terminology to Breast Imaging Reporting and Data System (BI-RADS), without assessing nodule vascularization or blood flow characteristics. The present results showed that Kwak TI-RADS is correlated with the FNAB results (i.e., cytological evaluation). Moreover, our results were consistent with the literature, suggesting that Kwak TI-RADS is a simple and reliable classification system that can be used in routine practice in different clinical settings. In this regard, Horvath et al., who were the first researchers to employ the TI-RADS, used similar US features in this system. They also examined the vascularization of nodules, unlike Kwak (3). Overall, assessment of nodule vascularization may be problematic, because it differs among users. Besides, its use has become more complicated over time, requiring device-dependent technical adjustments.
Moreover, in a study by Yoon et al., which compared six guidelines (SRU, National Comprehensive Cancer Network system, ATA system, French TI-RADS, Kim TI-RADS, and Kwak TI-RADS), the sensitivity of Kwak TI-RADS was the highest (98.8%) (19). Similar to the present study, the associations between malignancy and the five US findings, recommended by Kwak et al., were evaluated in their study, which were found to be significant (19). Moreover, in a comparative study by Gao et al., Kwak TI-RADS showed a higher diagnostic efficacy than other methods, with malignancy rates of 1.9%, 10.9%, 55.2%, 88.8%, and 87.1% reported in patients with TI-RADS 3, 4a, 4b, 4c, and 5 lesions, respectively. The malignancy rates for TI-RADS 3, 4a, 4b, and 4c were higher than our results and the rates reported by Kwak and colleagues; the high rate of malignancy was attributed to the high incidence of malignancy in their study population (9, 10).
According to Kwak TI-RADS, the probability of malignancy was as follows: TI-RADS 1, 0% (negative); TI-RADS 2, 0% (benign); TI-RADS 3, 1.7% (probably benign); TI-RADS 4a, 3.3% (low suspicion for malignancy); TI-RADS 4b, 9.2% (intermediate suspicion for malignancy); TI-RADS 4c, 44.4-72.4% (moderate concern, but not classic for malignancy); and TI-RADS 5, 87.5% (highly suggestive of malignancy) (9). TI-RADS 1 and 2 lesions were not included in our study. The malignancy rates were lower in patients classified as TI-RADS 3, TI-RADS 4a, and TI-RADS 4b (0.7%, 1.4%, and 5.9%, respectively), and the frequency of benign nodules was higher in our study compared to the study by Kwak and colleagues. It should be noted that an inclusive approach was adopted in our study to perform US-FNAB, even in patients without clear FNAB indications; therefore, the number of benign cases was relatively high in the present study. The number of benign cases undergoing surgery was also higher than their study, which might partly explain the higher frequency of FNAB requested by clinicians before surgery.
Consistent with the study by Kwak et al., the malignancy rates were 46.7% and 89.5% in patients classified as TI-RADS 4c and TI-RADS 5 in the current study, respectively. Besides, the rate of malignancy in TI-RADS 5 patients was similar to the rate reported by Gao et al. (9, 10). Based on the univariate analysis in the current study, the presence of a solid component, hypoechogenicity, microlobulated or irregular margins, microcalcifications, and a taller-than-wide shape significantly indicated malignancy; this finding is in line with the results reported by Kwak et al. (9). However, the results of our multivariate analysis were not compatible with the study by Kwak et al., because only hypoechogenicity and a taller-than-wide shape were significant indicators of malignancy.
Additionally, Shapira-Zaltsberg et al. used the Kwak TI-RADS classification for a group of pediatric patients and found different malignancy rates in the TI-RADS groups. The malignancy rates of TI-RADS 3, TI-RADS 4a, TI-RADS 4b, TI-RADS 4c, and TI-RADS 5 were 5.9%, 23.4%, 25.0%, 33.3%, and 66.7%, respectively. Compared to the study by Kwak et al., the malignancy rates of TI-RADS 3, TI-RADS 4a, and TI-RADS 4b were higher, while the malignancy rates of TI-RADS 4c and TI-RADS 5 were lower (20). Moreover, in a study by Seifert et al., the malignancy rates of TI-RADS 3, TI-RADS 4a, TI-RADS 4b, TI-RADS 4c, and TI-RADS 5 were 0%, 7%, 19%, 26%, and 33%, based on Kwak TI-RADS, respectively. The malignancy rates of TI-RADS 3, TI-RADS 4c, and TI-RADS 5 were lower compared to the study by Kwak et al., while the malignancy rates of TI-RADS 4a and TI-RADS 4b were higher (7). Although the ratio of malignancy increased with the TI-RADS category, the percentage of malignancy in their study was lower than the present study.
While nodule size is critical in the EU TI-RADS classification, in the present study, the nodule size was not considered as an indication of FNAB, similar to Kwak TI-RADS (21). The sizes of nodules included in our study was 1 cm or larger, based on Kwak TI-RADS. Also, because nodules are not classified by scoring in Kwak TI-RADS (22), we did not score the nodule size. Overall, in the present study, 38 (4.8%) out of 776 US-FNAB procedures were inadequate, and the failure rate was lower than the rates reported in the literature (23). The US-FNAB inadequacy rate was estimated at 15.1% in the study by Kwak et al., which is significantly higher than the literature and our study (9); this discrepancy may be due to the use of a smaller plastic syringe. Larger case-series and controlled studies are needed to further evaluate the causes of inadequate US-FNAB.
The most important limitations of this study included its single-center, retrospective design and the large number of benign cases. Also, patients with Bethesda III, IV, and V lesions were not included in our study, because repeat and/or follow-up FNABs were not examined; therefore, the characteristics of these patient groups were not analyzed. Besides, the low frequency of postoperative histopathological confirmation (n = 117, 18.2%) and the low frequency of malignant cases (n = 42, 6.5%) were limiting factors in our study. Because we did not have a homogeneous patient group, some of our results were inconsistent with the literature. Also, the low malignancy rates of TI-RADS 3, TI-RADS 4a, and TI-RADS 4b lesions might be attributed to the high number of benign cases in our study population. However, this does not diminish the significance of our findings and/or TI-RADS classification, as the malignancy rate significantly increased by using TI-RADS in our study, similar to BI-RADS.
In conclusion, US-FNAB is important in distinguishing malignant from benign nodules and preventing unnecessary surgical interventions. However, the clinicians’ decision to perform US-FNAB may lead to unnecessary FNAB procedures. Radiologists should consider the five mentioned US features (i.e., solid components, hypoechogenicity, microlobulation or irregular margins, microcalcifications, and a taller-than-wide shape) and use Kwak TI-RADS in the US report. The present results indicated that Kwak TI-RADS classification, which allows for US characterization of thyroid nodules, is correlated with the cytopathological results. Overall, the use of Kwak TI-RADS classification may increase communication between radiologists and clinicians. Also, with the widespread use of Kwak TI-RADS, it is possible to select patients who require FNAB more accurately. Since our results are compatible with the literature, they may be easily adopted in daily practice worldwide. Overall, the use of Kwak TI-RADS is recommended in the clinical setting.