1. Background
Chronic liver disease, which is known to have various etiologies, can result in hepatic fibrosis. The prevalence of non-alcoholic fatty liver disease (NAFLD) is estimated at 25%. NAFLD is rapidly becoming a major cause of chronic liver disease (1). It has been reported as the second most common cause of liver transplantation in the United States, with an ongoing increase in prevalence.(2)
Liver fibrosis is defined as the accumulation of extracellular matrix proteins, particularly collagen, which is most commonly associated with chronic liver diseases (3). Hepatic steatosis is described as the accumulation of fat within the cytoplasm of hepatocytes (4, 5). The most effective treatment for hepatic fibrosis is to manage the underlying cause of liver disease. Besides, antifibrotic treatments targeting hepatic stellate cells, as progenitors of hepatic fibrosis, and different key cytokines involved in fibrogenesis have been highlighted in different studies (6).
The main factor in the management of patients with chronic liver disease is monitoring hepatic fibrosis for progression or possible regression in case of effective treatment. Diagnosis in earlier stages is also crucial for management and improved outcomes. Liver biopsy, as the gold standard for the assessment of hepatic fibrosis, is an invasive method, associated with some complications (7, 8). Accordingly, non-invasive, reliable, simple, and cost-effective methods are needed. Transient elastography (TE) using a FibroScan system and shear wave elastography (SWE) using sonography have been introduced for evaluating fibrosis, while the controlled attenuation parameter (CAP) measured by FibroScan and the B-mode hepatorenal ratio measured by sonography have been introduced for evaluating hepatosteatosis (9-12).
2. Objectives
This study aimed to examine the concordance between different tools used for measuring liver fibrosis (TE vs. SWE) and hepatosteatosis (CAP vs. B-mode ratio) and to determine the effects of demographic variables on these measurements in patients, referred to the liver clinic of our university-affiliated center.
3. Patients and Methods
3.1. Participants
In this cross-sectional study, NAFLD patients, referred to the liver clinic of our center for a non-invasive assessment of hepatic fibrosis during March 2018 - 2019, were enrolled. The patients consented to undergo two methods of fibrosis and steatosis assessment (TE and CAP measurements by FibroScan and SWE and B-mode sonography). It should be noted that written consent was obtained from each participant before participation in the study. This study was conducted based on the Declaration of Helsinki. The university ethics committee approved the study protocol (ethics code: IR.sums.med.rec.1397.454). Patients with sonographic features of cirrhosis (ie, an irregular liver surface and lobar redistribution), multiple hepatic masses, or moderate to large ascites, as well as uncooperative patients during the tests, were excluded.
3.2. Procedures and Assessments
FibroScan was performed by a gastroenterologist in the liver clinic. In a supine position, the patient’s right arm was held upright to access the right hypochondrium. The optimal place for the probe was where the midaxillary vertical line crossed the xiphoidal horizontal line. A 5-MHz unidimensional M-probe was inserted 25 - 65 mm beneath the skin over a 4×1 cm region of interest (ROI); the mentioned method was repeated ten times. The median fibrosis (elasticity) score was presented in kilopascal (kPa) and classified based on the METAVIR score: F0-1, 5.5 (4.1 - 7.1) kPa; F2, 6.6 (4.8 - 9.6) kPa; F3, 10.3 (7.6 - 12.9) kPa; and F4, 30.8 (16.3 - 48) kPa. Moreover, the Brunt steatosis staging system was applied according to the percentage of fat accumulation in hepatocytes (S0, 0 - 10%; S1, 11 - 33%; S2, 34 - 66%; and S3, 67 - 100%), in addition to the median steatosis CAP score in dB/m.
SWE was performed by a staff radiologist using a SuperSonic device in the radiology clinic. The patients were instructed to fast for at least six hours. While they were in the left lateral position, their right arm was abducted. They were asked to hold their breath for at least six seconds for placing a 7-MHz concave probe on the right intercostal space. The best place for the probe was over the right lobe of the liver, where there was no sign of a large vessel or lesion, with an appropriate tissue resolution (distance to hepatic capsule ≥ 2 cm).
The fibrosis stage was measured after a homogeneous color mapping. This method was repeated ten times, and the median fibrosis (elasticity) score was recorded in kPa. The second heapatosteatosis assessment was the B-mode hepatorenal ratio measurement, which classified the patients into three groups: mild, < 1.49; 1.49 ≤ moderate < 1.86; and severe, ≥ 1.86. Besides, the conventional gray-scale sonographic data and demographic information, including the body mass index (BMI), sex, and age, were recorded for every patient.
3.3. Data Analysis
Statistical analysis was performed using SPSS Version 22.0 for Windows (released in 2013, IBM Corp., Armonk, NY, USA), as well as the Deducer Package powered by R programming language (Version 3.4.4 for Windows). Qualitative and quantitative variables were described using frequency (percentage) and mean ± standard deviation (SD) or median and interquartile range (ICR), respectively. They were also visualized by box plots and scatter plots.
Kolmogorov-Smirnov test was used to examine the distribution of variables, and non-parametric tests were used when there was no normal distribution. The groups were compared using Chi-square test for qualitative variables (male vs. female) and independent t-test or Mann-Whitney test for quantitative variables (BMI < 30 kg/m2 vs. BMI ≥ 30 kg/m2). Moreover, Pearson’s correlation coefficient test showed a linear correlation between the measurements of different quantitative methods for fibrosis and steatosis grading. P-value ≤ 0.05 was considered statistically significant.
4. Results
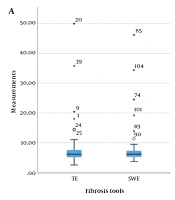
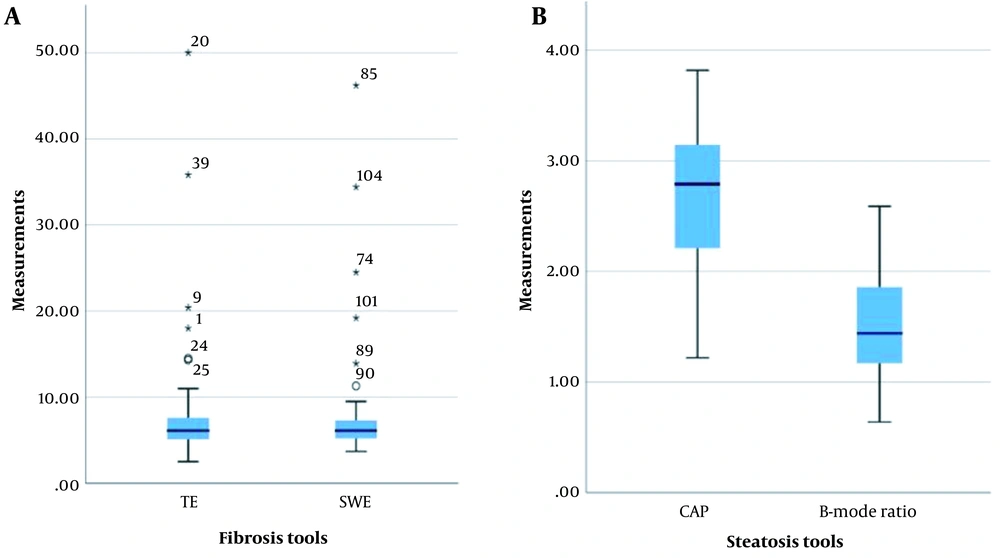
This study was performed on 65 patients, with the median age of 47 years (range, 41 - 55 years) and a male-to-female ratio of 1:13. The missing data were excluded from the study. The mean BMI of the patients was 27.3 kg/m2 (range: 23.6 - 29.6 kg/m2), suggesting that most of the patients were overweight. The patients’ demographic characteristics and the median TE, SWE, CAP, and B-mode ratio are presented in Table 1 and Figure 1.
| Variables | Values |
|---|---|
| Number | 65 |
| Age, median (ICR) | 47 (41 - 55) |
| Sex, No. (%) | |
| Female | 30 (46.2) |
| Male | 34 (52.3) |
| Missing | 1 (1.5) |
| BMI, median (ICR) | 27.3 (23.6 - 29.6) |
| TE, median kPa (ICR) | 6.1 (5.1 - 7.6) |
| SWE, median kPa (ICR) | 6.1 (5.2 - 7.3) |
| CAP, median dB/m (ICR) | 279 (227.5 - 313.25) |
| B-mode ratio, median score (ICR) | 1.44 (1.17 - 1.86) |
Abbreviations: BMI, body mass index; CAP, controlled attenuation parameter; ICR, interquartile range; SWE, shear wave elastography; TE, transient elastography.
The box plot of median measurements of fibrosis (A) and steatosis (B) (central horizontal line: Median; upper and lower horizontal lines: First and third quartiles; and corner horizontal lines: minimum and maximum); asterisk indicates the outliers. The CAP measurements are divided by 100 to reach a homogenous scale (CAP, Controlled attenuation parameter; SWE, Shear wave elastography; TE, Transient elastography).
The fibrosis staging tools, TE, and SWE yielded perfectly correlated measurements (r = 0.9538; 95% confidence interval (CI): 0.9252 - 0.9717) (Figure 2A). Overall, 54 (83.1%) patients had fibrosis scores ≤ 2. The CAP and B-mode ratio were moderately correlated for steatosis grading (r = 0.429; 95% CI: 0.2048 - 0.6104) (Figure 2B), especially for moderate and severe hepatosteatosis. Compared to CAP and gray-scale sonography, the B-mode ratio classified a greater proportion of the participants into mild steatosis (Table 2). There was no significant correlation between other variables (ie, sex, age, and BMI) and different staging tools (P > 0.05). Nevertheless, a mild correlation was found between BMI and CAP (P = 0.005) (Table 3).
Linear correlations (A) between TE and SWE and (B) between CAP and B-mode ratio (each dot is representative of a case; the green area represents 95% confidence interval [CIs]). Red numbers represent the Pearson’s correlation coefficients. CAP, Controlled attenuation parameter; SWE, Shear wave elastography; TE, Transient elastography.
| Hepatic steatosis | |||
|---|---|---|---|
| Steatosis grade | CAP (Brunt score) | Gray-scale sonography | B-mode ratio |
| S0 | 16 (24.6) | 15 (23.1) | 6 (9.2) |
| S1 | 8 (12.3) | 8 (12.3) | 21 (32.3) |
| S2 | 20 (30.8) | 29 (44.6) | 17 (26.2) |
| S3 | 21 (32.3) | 13 (20) | 21 (32.3) |
Abbreviation: CAP, controlled attenuation parameter.
a Values are expressed as Number (percentage)
| Sex (Mean ± SD) | P-value a | BMI a | P-value a | |||
|---|---|---|---|---|---|---|
| Male | Female | < 30 | ≥ 30 | |||
| TE | 8.75 ± 8.27 | 7.21 ± 5.87 | 0.389 | 11.14 ± 11.92 | 7.1 ± 1.21 | 0.235 |
| SWE | 8.7 ± 8.27 | 7.11 ± 5.37 | 0.34 | 10.78 ± 11.34 | 7.12 ± 4.64 | 0.258 |
| CAP | 263.21 ± 63.69 | 274.86 ± 72.07 | 0.502 | 310.21 ± 71.85 | 257.1 ± 61.2 | 0.005 b |
| B-mode ratio | 1.46 ± 0.46 | 1.58 ± 0.5 | 0.298 | 1.72 ± 0.55 | 1.46 ± 0.44 | 0.118 |
Abbreviations: CAP, controlled attenuation parameter; SWE, shear wave elastography; TE, transient elastography; BMI, body mass index; SD, standard deviation.
a Independent t-test
b Mann-Whitney test
5. Discussion
Although liver biopsy is the gold standard for hepatic fibrosis staging, it is an invasive method, associated with rare, but serious potential risks of bleeding, damage to the adjacent organs, and even death due to complications. Besides, it is not the most cost-effective option, and resources, as well as expertise, are needed for sampling. Also, an expert pathologist must be present for the interpretation of histological findings. Moreover, liver biopsy is subject to sampling errors, as histological assessment is performed on a very small liver volume (1/50,000); also, because the liver may not be homogenously involved in pathology, the assessment may not be precise (13). Therefore, with a global increase in the prevalence of chronic liver diseases, reliable and non-invasive methods of liver disease staging are needed.
5.1. Fibrosis
In the present study, the two non-invasive methods of TE and SWE were strongly correlated for fibrosis staging. This finding is consistent with several previous studies, including those conducted by Bende et al. (r = 0.83) (14), Kircheis et al. (r = 0.92) (15), Zeng et al. (r = 0.835) (16), and Paul et al. (r = 0.33) (17). Overall, there is no preference for the use of these two methods in fibrosis assessment, and the choice depends on factors unrelated to internal validity, including the operator’s experience, cost-effectiveness, and availability (18). However, it seems that SWE has several advantages over TE.
SWE is a numeric and color-coded modality, in which the operator can choose the most homogenous ROI. Besides, it is a real-time modality; therefore, large vessels and the liver capsule can be avoided (14). In this regard, a meta-analysis by Bota et al. (19) showed that SWE fails less than TE (2.1% vs. 6.6%) in providing reliable measurements. Besides, the SWE results are more independent of steatosis than TE (20-24). Also, SWE is more available than TE. Because it is performed by an ultrasound machine, hepatosteatosis measurements based on gray-scale sonography or B-mode hepatorenal ratio can be performed simultaneously with the assessment of hepatobiliary imaging features. However, it should be noted that SWE is operator-dependent and should be performed by a trained ultrasonographer; besides, the patient’s cooperation is essential, and the examination time is longer.
A high BMI (> 28 kg/m2) or a small intercostal space can affect both TE and SWE results (25, 26). In the present study, the effect of BMI on the measurements was not significant, which is similar to the finding reported by Cassinotto et al. (27). Similar to our results, in another study, Cassinotto et al. (28) showed that SWE and TE results were not affected by age or sex. It should be noted that several studies have reported older age as a confounding factor for TE and SWE (29-31). In the current study, most elderly patients were excluded due to inadequate cooperation for performing the tests; therefore, the study was performed on a relatively young population with the median age of 47 years.
The majority of published studies support the higher accuracy of non-invasive methods in the detection of more advanced fibrosis (32-35). The present study mostly included patients with non-advanced stages of fibrosis, with more than 80% of patients having stage 2 fibrosis or lower. The main reason is that sonographic features of cirrhosis and the presence of ascites were among the exclusion criteria of this study. Therefore, further studies on a larger number of patients with hepatic fibrosis stages 3 and 4 are needed to assess the correlation of these two modalities in advanced stages of fibrosis. Finally, the recommended cutoff values for fibrosis staging by TE showed moderate concordance with SWE, and they should not be used interchangeably. It is recommended that future studies use liver biopsy as the gold standard reference to assess the internal validity, sensitivity, and specificity of both modalities and the SWE cutoff points.
5.2. Hepatosteatosis
Gray-scale ultrasound is commonly used for hepatosteatosis screening. However, it has some limitations, such as interference of liver fibrosis and tissue inflammation with the accuracy of results. Another limitation of gray-scale ultrasound is a low sensitivity of about 55% for lower grades of steatosis. Also, high BMI and subcutaneous fat content cause technical difficulties and signal attenuation, resulting in decreased accuracy (12, 36-38). Besides, this modality is a subjective, operator-dependent visual tool with interobserver variability (39). Accordingly, more reliable non-invasive techniques are essential for a precise assessment of hepatosteatosis and its severity. The B-mode hepatorenal ratio and CAP are new parameters for the measurement of hepatostaosis, with possibly higher accuracies than gray-scale ultrasound.
Not many studies have compared the CAP and B-mode ratio. In a study by Fujiwara et al. (40), similar to our findings, the correlation between these parameters was high (r = 0.81). In the present study, almost two-thirds of the patients were classified into moderate or severe hepatosteatosis groups, based on all three methods. It seems that the B-mode ratio method can detect minimal steatosis more efficiently than other methods, as mild steatosis was detected in a larger number of patients compared to gray-scale sonography or CAP. Therefore, the B-mode ratio can be the preferred option for the early detection of fatty liver.
There are also fewer findings supporting the superiority of the B-mode ratio over other methods. In the present study, none of the studied demographic variables influenced the B-mode ratio measurements, while BMI interacted with the CAP measurements. In the B-mode ratio method, we can map the liver tissue, whereas the CAP method is limited due to the interference of extrahepatic structures with the measurements because of a fixed ROI. Additionally, the B-mode ratio can be measured by a single probe (B-mode probe), while for CAP measurements in obese cases, it may be essential to replace the M-probe with an XL probe (41, 42).
In conclusion, similar to previous research, non-invasive modalities were strongly correlated for the assessment of fibrosis and hepatosteatosis in the present study. It was found that factors, such as age, sex, and BMI, did not interfere with the tests results, although BMI was a confounding factor for the CAP results. Overall, selection of the best non-invasive method for the assessment of hepatic fibrosis and steatosis depends on factors other than internal validity. In a local setting, SWE and B-mode ratio measurement may be preferred for detecting fibrosis and hepatosteatosis, respectively.
The limitation of this study was the lack of histopathological results of liver biopsies as a gold standard for comparison. Future studies are recommended to use liver histology as a gold standard to compare the diagnostic results of different hepatic fibrosis and steatosis grading tools.
![Linear correlations (A) between TE and SWE and (B) between CAP and B-mode ratio (each dot is representative of a case; the green area represents 95% confidence interval [CIs]). Red numbers represent the Pearson’s correlation coefficients. CAP, Controlled
attenuation parameter; SWE, Shear wave elastography; TE, Transient elastography. Linear correlations (A) between TE and SWE and (B) between CAP and B-mode ratio (each dot is representative of a case; the green area represents 95% confidence interval [CIs]). Red numbers represent the Pearson’s correlation coefficients. CAP, Controlled
attenuation parameter; SWE, Shear wave elastography; TE, Transient elastography.](https://services.brieflands.com/cdn/serve/3170b/ac8fba93e5be0921eea41786e84b358c147ffef9/iranjradiol-112589-i002-F2-preview.webp)