1. Background
Digital mammography (DM) is the most important radiological screening and diagnostic tool, which has been shown to increase the survival of breast cancer patients (1). While the sensitivity of DM is high in fatty breasts, it decreases to 30% in dense breasts (2). In DM, dense fibroglandular tissue may lead to false negative results by superimposing on the lesion margins (2). To eliminate superimposing the normal fibroglandular tissue, an adjunctive radiological method, such as ultrasonography or digital breast tomosynthesis (DBT), is commonly applied. DBT is a recently developed modality that yields multiple mammographic images and combines them with an algorithm to create a three-dimensional image. By allowing the breast tissue examination in different sections, DBT decreases the superimposition of fibroglandular tissue and improves the detection rate of breast cancer significantly (3).
Breast cancer is a morphologically heterogeneous disease with various histopathological parameters and multiple receptors in its biological profile. Differences in the gene expression profile of breast tumors may be responsible for differences in the prognosis of patients. In the latest edition of breast tumor classification by the World Health Organization (WHO), it has been declared that breast cancer is heterogeneous at the molecular level (4). Estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 antigen are commonly used for the molecular classification of breast tumors. Currently, five widely accepted molecular subgroups have been identified: (1) luminal A; (2) luminal B, HER2-positive; (3) luminal B, HER2-negative; (4) HER2 positive; and (5) triple negative (5).
Major efforts have been made, especially in the last few years, to classify breast tumors at the molecular level and find more effective treatments (6). Besides, detection of breast cancer molecular subtypes using radiological modalities is of particular importance for the early treatment of breast cancer. Therefore, early detection and treatment can provide long-term survival advantages (7). Although many studies have investigated the relationship between the morphological features of tumors detected by DM and the molecular subgroups of breast cancer, few studies have examined the relationships between lesion subgroups in DBT, which is a more effective modality for dense breasts.
2. Objectives
This study aimed to analyze the morphological features of invasive breast cancer on DM and DBT, to investigate the contribution of DBT to DM, to examine the association of DBT findings with the pathological molecular subtypes, Bloom-Richardson grade (BRG), and Ki-67 index, and finally, to investigate the effect of breast parenchyma density on the relationship between DBT findings and hormone receptor status.
3. Patients and Methods
A total of 36 patients with histopathologically proven malignant lesions were evaluated in this study. The histopathological results were obtained using Tru-cut biopsy or mastectomy. The exclusion criteria were as follows: (1) undergoing surgery or biopsy; (2) having other formerly known malignancies; and (3) receiving neoadjuvant chemotherapy.
The pathological subgroups and BRG were based on the fifth edition of the WHO guidelines. The luminal A subtype was defined as ER positivity, PR positivity, low Ki-67 index, and if applicable, multigene expression and low risk of recurrence. Although the luminal B, HER2-negative subtype was defined as being ER positive and HER2 negative, it was associated with a high Ki-67 index, low or negative PR, multigene expression, and a high recurrence risk. Moreover, in the luminal B, HER2-positive subtype, ER positivity and HER2 overexpression or amplification are essential. Also, the luminal B, HER2-positive subtype is defined as a high Ki-67 index and PR receptor positivity. In the HER2-positive subtype, the overexpression or amplification of HER2 and loss of ER and PR are essential. Finally, the triple-negative subtype is characterized by negative ER, PR, and HER2. In this classification system, a high Ki-67 index is defined as ≥ 20%, and ER and PR positivity is defined as 1%.
All patients underwent DM and DBT. A commercially available device (Mammomat Inspiration, Siemens, Erlangen, Germany) was used for all DM examinations. Also, DBT images were acquired using a Giotto Breast Tomosynthesis System (IMS, Bologna, Italy). The DM and DBT images were available in our local database. Mammography (MG) followed by DBT was performed for the patients. The patients’ images were evaluated by two radiologists, one of whom was specialized in breast radiology.
The breast density was classified as a, b, c, and d on MG. This classification was based on the 2013 American College of Radiology (ACR) breast atlas (8). There were two main categories of breast tissue: (1) non-dense (a and b); and (2) dense (c and d). According to the ACR classification, the shape of masses was divided into oval, round, and irregular. The margin features of the lesions were designated as circumscribed, obscured, microlobulated, indistinct, and spiculated. Also, the lesion density was determined as high density, isodensity, and low density in each modality (DM and DBT).
Moreover, the presence of microcalcifications was investigated in this study. If there was any microcalcification, it was divided into groups of typically benign (punctate) and suspicious morphology (amorphous, coarse heterogeneous, fine pleomorphic, and fine linear or branching), according to the microcalcification morphology. Besides, the microcalcification distribution was divided into categories of regional, grouped, linear, and segmental.
Additionally, architectural distortion, intramammary lymph nodes, skin changes, and nipple retraction were assessed in the present study. Tumor size, number of microcalcifications, and number of foci (foci with similar characteristics, but located in different points from the main lesion) were determined for each modality. Each of these previously measured morphological features was first evaluated by DM and then DBT, and the DM and DBT findings were compared. Moreover, the relationship between DBT findings and hormone receptor status was investigated, and the effect of density was assessed. Also, the relationship between DBT findings and molecular classification and BRG was determined.
This study was conducted based on the ethical standards, outlined in the Declaration of Helsinki of the World Medical Association (WMA). The study protocol was approved by our institutional review board. The study was carried out after an institutional ethical clearance was obtained.
3.1. Statistical Analysis
All statistical analyses were performed in R 3.6.0 (www.r-project.com). The Anderson-Darling test and Q-Q plot were used to examine the normal distribution of data. Continuous variables are presented as mean ± standard deviation (minimum/maximum) and compared using Student’s t-test. Categorical variables are described as number (n) and percentage (%) and compared using chi-square test. Moreover, to determine the superiority of DBT to DM, the interval likelihood ratio, sensitivity, and specificity were calculated. Besides, alluvial plots were drawn to show the distribution of PR subtypes in dense breasts according to skin changes, mass shape, and nipple retraction. A P-value less than 0.05 was considered statistically significant.
4. Results
The mean age of the patients (n = 36) was 53 years. Overall, 63% of the patients had lesions in the left breast, and 66.7% of the patients showed a dense parenchymal pattern. ER was positive in 31 (86.1%) patients, PR was positive in 29 (80.6%) patients, and HER2 was negative in 20 (55.6%) patients. Based on the findings, in 20 (55.6%) patients, the Ki-67 index was ≥ 20. According to the BRG system, 18 (50%) patients were classified as grade 3, and four patients were in the unclassified group. As for the molecular classification, luminal B, HER2-positive subtype was detected in 11 (30.6%) patients; it was the most commonly seen subgroup in the present study (Table 1).
| Characteristics | Number of breasts (percentage) |
|---|---|
| Age (y), mean ± SD (min - max) | 53.94 ± 9.08 (35 - 75) |
| Side | |
| Right | 13 (36.1) |
| Left | 23 (63.9) |
| Density pattern | |
| B | 12 (33.3) |
| C | 21 (58.3) |
| D | 3 (8.3) |
| Breast density | |
| Non-dense | 12 (33.3) |
| Dense | 24 (66.7) |
| ER | |
| Negative | 5 (13.9) |
| Positive | 31 (86.1) |
| PR | |
| Negative | 7 (19.4) |
| Positive | 29 (80.6) |
| HER2 | |
| Negative | 20 (55.6) |
| Positive | 16 (44.4) |
| Ki-67 index | |
| Low (< 20%) | 16 (44.4) |
| High (≥ 20%) | 20 (55.6) |
| BRG | |
| Grade 1 | 2 (5.6) |
| Grade 2 | 12 (33.3) |
| Grade 3 | 18 (50) |
| No grading (not possible) | 4 (11.1) |
| Molecular classification | |
| Luminal A subtype | 8 (22.2) |
| Luminal B subtype, HER2+ | 11 (30.6) |
| Luminal B subtype, HER2– | 6 (16.7) |
| HER2+ | 2 (5.6) |
| Triple-negative subtype | 3 (8.3) |
| No molecular classification | 6 (16.7) |
The Clinical and Pathological Features of Breast Cancer on DBT a
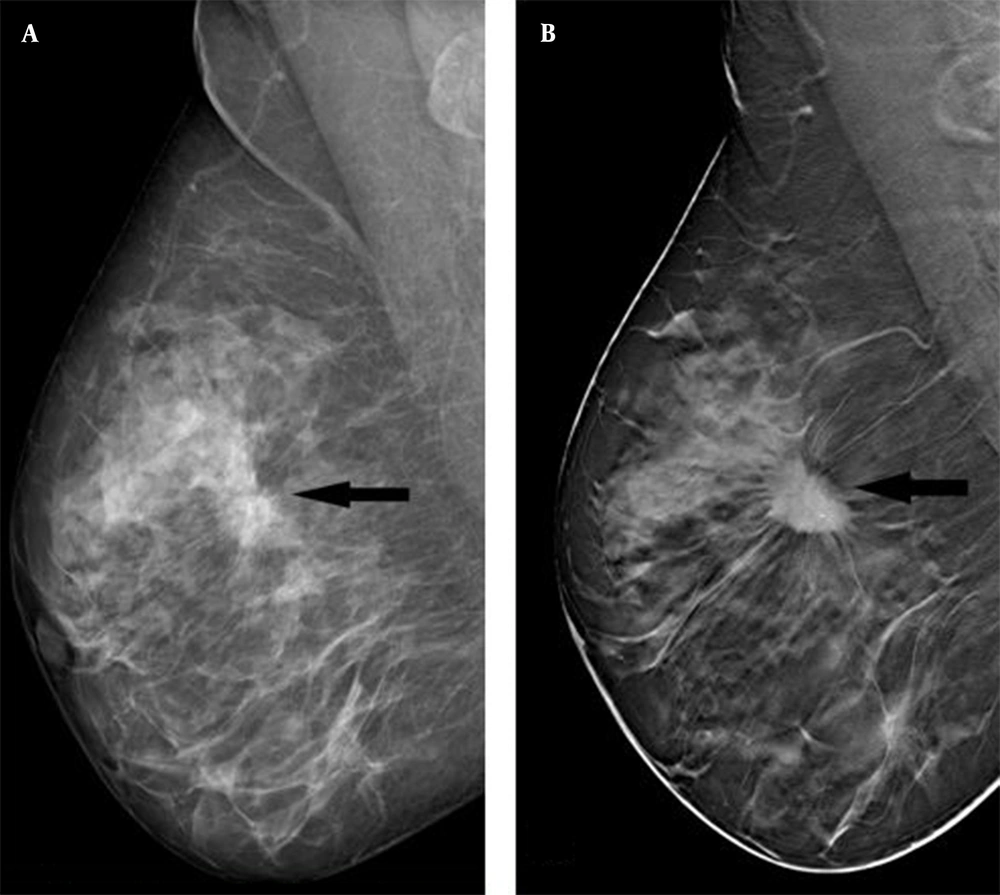
Based on the comparison of DM and DBT findings, spiculated margins, tumor density, architectural distortion, and microcalcifications were significantly higher in DBT. The mass lesion with indistinct margins on DM was seen as a lesion with spiculated margins on DBT (P < 0.001) (Figure 1A and B). Spiculated margins, high density, and architectural distortions were strong predictors of malignant tumors in DBT, as the likelihood ratio was 3.63 (1.92 - 6.82), 2.14 (1.38 - 3.31), and 1.39 (1.06 - 1.83), respectively, and sensitivity was estimated at 80.5, 83.3, and 88.8%, respectively. The majority of masses showed high densities on DBT (P < 0.001). The detection rate of architectural distortions on DBT was significantly higher than DM (P = 0.013). DBT was superior to DM in displaying the number of microcalcifications and satellite foci; however, the difference was not statistically significant (Table 2 and Figure 2).
| Variables | MG (n = 36) | DBT (n = 36) | Total | P-Value |
|---|---|---|---|---|
| Mass shape | ns | |||
| Oval | 1 (2.8) | 1 (2.8) | 2 (2.8) | |
| Round | 12 (33.3) | 7 (19.4) | 19 (26.4) | |
| Irregular | 23 (63.9) | 28 (77.8) | 51 (70.8) | |
| Mass margin | < 0.001 | |||
| Circumscribed | 1 (2.8) | 0 (0) | 1 (1.4) | |
| Obscured | 6 (16.7) | 0 (0) | 6 (8.3) | |
| Microlobulated | 3 (8.3) | 2 (2) | 5 (6.9) | |
| Indistinct | 18 (50) A | 5 (13.9) B | 23 (31.9) | |
| Spiculated | 8 (22.2) A | 29 (80.6) B | 37 (51.4) | |
| Density | < 0.001 | |||
| High density | 14 (38.9) A | 30 (83.3) B | 44 (61.1) | |
| Isodensity | 21 (58.3) A | 6 (16.7) B | 27 (37.5) | |
| Low density | 1 (2.8) | 0 (0) | 1 (1.4) | |
| Microcalcification | 0.002 | |||
| No | 23 (63.9) A | 10 (27.8) B | 33 (45.8) | |
| Yes | 13 (36.1) A | 26 (72.2) B | 39 (54.2) | |
| Calcification morphology | ns | |||
| Punctate | 2 (12.5) | 1 (4.5) | 3 (7.9) | |
| Amorphous | 11 (68.8) | 16 (72.7) | 27 (71.1) | |
| Pleomorphic | 3 (18.8) | 5 (22.7) | 8 (21.1) | |
| Distribution of microcalcifications | ns | |||
| Regional | 2 (12.5) | 3 (13.6) | 5 (13.2) | |
| Grouped | 11 (68.8) | 16 (72.7) | 27 (71.1) | |
| Linear | 0 (0) | 2 (9.1) | 2 (5.3) | |
| Segmental | 3 (18.8) | 1 (4.5) | 4 (10.5) | |
| Architectural distortion | 0.013 | |||
| No | 13 (36.1) A | 4 (11.1) B | 17 (23.6) | |
| Yes | 23 (63.9) A | 32 (88.9) B | 55 (76.4) | |
| Intramammary lymph nodes | ns | |||
| No | 32 (88.9) | 27 (75) | 59 (81.9) | |
| Yes | 4 (11.1) | 9 (25) | 13 (18.1) | |
| Skin changes | ns | |||
| No | 28 (77.8) | 28 (77.8) | 56 (77.8) | |
| Yes | 8 (22.2) | 8 (22.2) | 16 (22.2) | |
| Nipple retraction | ns | |||
| No | 27 (75) | 27 (75) | 54 (75) | |
| Yes | 9 (25) | 9 (25) | 18 (25) | |
| Tumor diameter, mean ± SD | 22.17 ± 13.67 | 25.08 ± 12.17 | ns | |
| Number of microcalcifications | 7.72 ± 12.09 | 10.97 ± 13.03 | 0.276 | |
| Number of foci | 0.22 ± 0.59 | 0.92 ± 1.36 | 0.007 |
Regarding the association of DBT findings with the hormone receptor status and Ki-67 proliferation index, an irregular mass shape was mostly observed in PR-positive patients (89.7%), while a round mass shape was more common in PR-negative patients (57%) (P = 0.003). In PR-negative patients, skin changes and nipple retraction were more frequent compared to PR-positive patients (P = 0.03 for skin changes and P = 0.049 for nipple retraction). However, no significant association was found between DBT findings and other receptors or Ki-67 proliferation index (Table 3). Regarding the effect of density on the significant relationship between the PR status and DBT findings, a significant relationship was only found in the dense group (P = 0.006 for mass shape; P = 0.038 for skin changes; and P = 0.038 for nipple retraction). Nevertheless, no significant correlation was found in the non-dense group.
| Variables | ER | PR | HER2 | Ki-67 index | ||||
|---|---|---|---|---|---|---|---|---|
| – (n = 5) | + (n = 31) | – (n = 7) | + (n = 29) | – (n = 20) | + (n = 16) | Low (n = 16) | High (n = 20) | |
| Mass shape | ||||||||
| Oval | 0 (0) | 1 (3.2) | 1 (14.3) | 0 (0) | 0 (0) | 1 (6.3) | 0 (0) | 1 (5) |
| Round | 3 (60) | 4 (12.9) | 4 (57.1) | 3 (10.3) | 5 (25) | 2 (12.5) | 3 (18.8) | 4 (20) |
| Irregular | 2 (40) | 26 (83.9) | 2 (28.6) | 26 (89.7) | 15 (75) | 13 (81.3) | 13 (81.3) | 15 (75) |
| P-value | ns | 0.003 | ns | ns | ||||
| Mass margin | ||||||||
| Microlobulated | 0 (0) | 2 (6.5) | 0 (0) | 2 (6.9) | 1 (5) | 1 (6.3) | 1 (6.3) | 1 (5) |
| Indistinct | 0 (0) | 5 (16.1) | 1 (14.3) | 4 (13.8) | 1 (5) | 4 (25) | 2 (12.5) | 3 (15) |
| Spiculated | 5 (100) | 24 (77.4) | 6 (85.7) | 23 (79.3) | 18 (90) | 11 (68.8) | 13 (81.3) | 16 (80) |
| P-value | ns | ns | ns | ns | ||||
| Density | ||||||||
| High density | 5 (100) | 25 (80.6) | 7 (100) | 23 (79.3) | 16 (80) | 14 (87.5) | 12 (75) | 18 (90) |
| Isodensity | 0 (0) | 6 (19.4) | 0 (0) | 6 (20.7) | 4 (20) | 2 (12.5) | 4 (25) | 2 (10) |
| P-value | ns | ns | ns | ns | ||||
| Microcalcifications | ||||||||
| No | 2 (40) | 8 (25.8) | 3 (42.9) | 7 (24.1) | 4 (20) | 6 (37.5) | 2 (12.5) | 8 (40) |
| Yes | 3 (60) | 23 (74.2) | 4 (57.1) | 22 (75.9) | 16 (80) | 10 (62.5) | 14 (87.5) | 12 (60) |
| P-value | ns | ns | ns | ns | ||||
| Calcification morphology | ||||||||
| Punctate | 1 (33.3) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 1 (8.3) |
| Amorphous | 2 (66.7) | 14 (73.7) | 3 (75) | 13 (72.2) | 7 (70) | 9 (75) | 8 (80) | 8 (66.7) |
| Pleomorphic | 0 (0) | 5 (26.3) | 0 (0) | 5 (27.8) | 3 (30) | 2 (16.7) | 2 (20) | 3 (25) |
| P-value | ns | ns | ns | ns | ||||
| Distribution of microcalcifications | ||||||||
| Regional | 1 (33.3) | 2 (10.5) | 2 (50) | 1 (5.6) | 1 (10) | 2 (16.7) | 1 (10) | 2 (16.7) |
| Grouped | 2 (66.7) | 14 (73.7) | 2 (50) | 14 (77.8) | 8 (80) | 8 (66.7) | 8 (80) | 8 (66.7) |
| Linear | 0 (0) | 2 (10.5) | 0 (0) | 2 (11.1) | 0 (0) | 2 (16.7) | 0 (0) | 2 (16.7) |
| Segmental | 0 (0) | 1 (5.3) | 0 (0) | 1 (5.6) | 1 (10) | 0 (0) | 1 (10) | 0 (0) |
| P-value | ns | ns | ns | ns | ||||
| Architectural distortion | ||||||||
| No | 0 (0) | 4 (12.9) | 1 (14.3) | 3 (10.3) | 2 (10) | 2 (12.5) | 3 (18.8) | 1 (5) |
| Yes | 5 (100) | 27 (87.1) | 6 (85.7) | 26 (89.7) | 18 (90) | 14 (87.5) | 13 (81.3) | 19 (95) |
| P-value | ns | ns | ns | ns | ||||
| Intramammary lymph nodes | ||||||||
| No | 5 (100) | 22 (71) | 7 (100) | 20 (69) | 16 (80) | 11 (68.8) | 14 (87.5) | 13 (65) |
| Yes | 0 (0) | 9 (29) | 0 (0) | 9 (31) | 4 (20) | 5 (31.3) | 2 (12.5) | 7 (35) |
| P-value | ns | ns | ns | ns | ||||
| Skin changes | ||||||||
| No | 2 (40) | 26 (83.9) | 3 (42.9) | 25 (86.2) | 14 (70) | 14 (87.5) | 11 (68.8) | 17 (85) |
| Yes | 3 (60) | 5 (16.1) | 4 (57.1) | 4 (13.8) | 6 (30) | 2 (12.5) | 5 (31.3) | 3 (15) |
| P-value | ns | 0.030 | ns | ns | ||||
| Nipple retraction | ||||||||
| No | 2 (40) | 25 (80.6) | 3 (42.9) | 24 (82.8) | 14 (70) | 13 (81.3) | 11 (68.8) | 16 (80) |
| Yes | 3 (60) | 6 (19.4) | 4 (57.1) | 5 (17.2) | 6 (30) | 3 (18.8) | 5 (31.3) | 4 (20) |
| P-value | ns | 0.049 | ns | ns | ||||
The Relationship Between DBT Findings and Hormone Receptor Status and Ki-67 Index
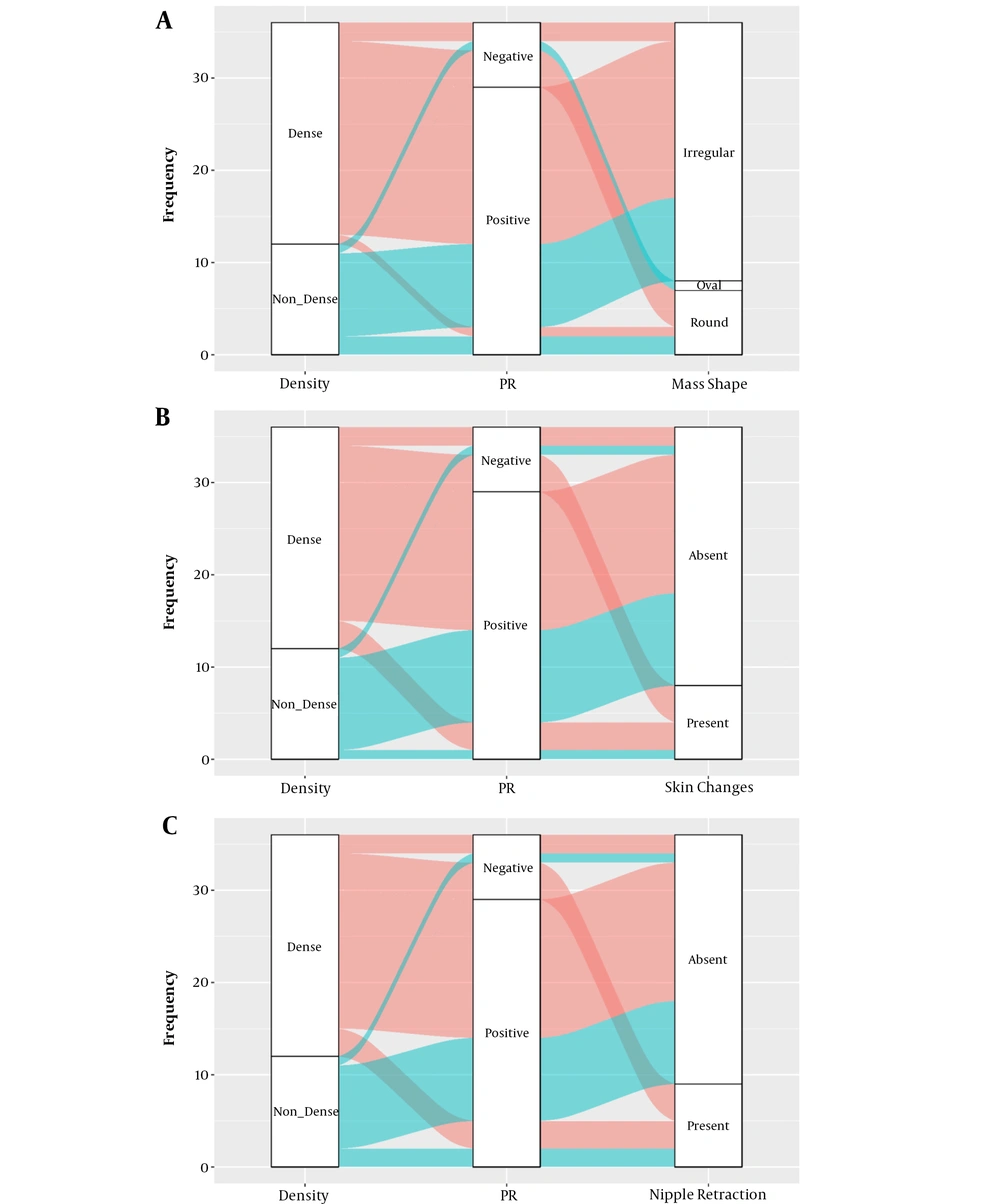
In the dense group, 5.6% of PR-positive patients had round-shaped tumors, while 94% of them had irregular tumors. In the dense group, 66.7% of PR-negative patients had round tumors, whereas 33.3% of them had irregular ones. While skin changes and nipple retraction were observed in 66.7% of PR-negative patients in the dense group, 83.3% of PR-positive patients did not show such findings (Table 4). The figurative alluvial plots of this table are presented below (Figure 3A - C).
| Variables | Non-dense breasts | Dense breasts | ||
|---|---|---|---|---|
| PR– (n = 1) | PR+ (n = 11) | PR– (n = 6) | PR+ (n = 18) | |
| Mass shape | ||||
| Oval | 1 (100) | 0 (0) | - | - |
| Round | 0 (0) | 2 (18.2) | 4 (66.7) | 1 (5.6) |
| Irregular | 0 (0) | 9 (81.8) | 2 (33.3) | 17 (94.4) |
| P-value | ns | 0.006 | ||
| Skin changes | ||||
| No | 1 (100) | 10 (90.9) | 2 (33.3) | 15 (83.3) |
| Yes | 0 (0) | 1 (9.1) | 4 (66.7) | 3 (16.7) |
| P-value | ns | 0.038 | ||
| Nipple retraction | ||||
| No | 1 (100) | 9 (81.8) | 2 (33.3) | 15 (83.3) |
| Yes | 0 (0) | 2 (18.2) | 4 (66.7) | 3 (16.7) |
| P-value | ns | 0.038 | ||
Regarding the association of BRG with DBT findings, a significant relationship was found only between the mass density and grade. Accordingly, tumors with higher grades were more likely to be accompanied by a high density (P = 0.032). Considering the association of BRG with other DBT findings, no significant difference was detected (Table 5). Besides, regarding the relationship between molecular classification and DBT findings, skin changes and nipple retraction were significantly more frequent in triple-negative masses compared to other subtypes (P = 0.011 for skin changes and P = 0.016 for nipple retraction). In other subtypes, no significant difference was detected (Table 6).
| Variables | BRG grade | Total | P-value | |||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade cannot be determined | |||
| Mass shape | ns | |||||
| Oval | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 1 (2.8) | |
| Round | 0 (0) | 1 (8.3) | 5 (27.8) | 1 (25) | 7 (19.4) | |
| Irregular | 2 (100) | 10 (83.3) | 13 (72.2) | 3 (75) | 28 (77.8) | |
| Mass margin | ns | |||||
| Microlobulated | 0 (0) | 1 (8.3) | 0 (0) | 1 (25) | 2 (5.6) | |
| Indistinct | 0 (0) | 1 (8.3) | 3 (16.7) | 1 (25) | 5 (13.9) | |
| Spiculated | 2 (100) | 10 (83.3) | 15 (83.3) | 2 (50) | 29 (80.6) | |
| Density | 0.032 | |||||
| High density | 0 (0) | 11 (91.7) | 16 (88.9) | 3 (75) | 30 (83.3) | |
| Isodensity | 2 (100) | 1 (8.3) | 2 (11.1) | 1 (25) | 6 (16.7) | |
| Microcalcification | ns | |||||
| No | 0 (0) | 4 (33.3) | 6 (33.3) | 0 (0) | 10 (27.8) | |
| Yes | 2 (100) | 8 (66.7) | 12 (66.7) | 4 (100) | 26 (72.2) | |
| Calcification morphology | ns | |||||
| Punctate | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 1 (4.5) | |
| Amorphous | 1 (100) | 5 (83.3) | 7 (58.3) | 3 (100) | 16 (72.7) | |
| Pleomorphic | 0 (0) | 1 (16.7) | 4 (33.3) | 0 (0) | 5 (22.7) | |
| Distribution of microcalcifications | ns | |||||
| Regional | 0 (0) | 0 (0) | 3 (25) | 0 (0) | 3 (13.6) | |
| Grouped | 1 (100) | 6 (100) | 6 (50) | 3 (100) | 16 (72.7) | |
| Linear | 0 (0) | 0 (0) | 2 (16.7) | 0 (0) | 2 (9.1) | |
| Segmental | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 1 (4.5) | |
| Architectural distortion | ns | |||||
| No | 1 (50) | 1 (8.3) | 1 (5.6) | 1 (25) | 4 (11.1) | |
| Yes | 1 (50) | 11 (91.7) | 17 (94.4) | 3 (75) | 32 (88.9) | |
| Intramammary lymph nodes | ns | |||||
| No | 1 (50) | 9 (75) | 13 (72.2) | 4 (100) | 27 (75) | |
| Yes | 1 (50) | 3 (25) | 5 (27.8) | 0 (0) | 9 (25) | |
| Skin changes | ns | |||||
| No | 2 (100) | 9 (75) | 13 (72.2) | 4 (100) | 28 (77.8) | |
| Yes | 0 (0) | 3 (25) | 5 (27.8) | 0 (0) | 8 (22.2) | |
| Nipple retraction | ns | |||||
| No | 2 (100) | 10 (83.3) | 11 (61.1) | 4 (100) | 27 (75) | |
| Yes | 0 80) | 2 (16.7) | 7 (38.9) | 0 (0) | 9 (25) | |
| Variables | Molecular classification | P-value | |||||
|---|---|---|---|---|---|---|---|
| Luminal A subtype | Luminal B subtype, HER2+ | Luminal B subtype, HER2– | HER2+ | Triple-negative subtype | No molecular classification | ||
| Mass shape | ns | ||||||
| Oval | 0 (0) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Round | 0 (0) | 1 (9.1) | 1 (16.7) | 1 (50) | 2 (66.7) | 2 (33.3) | |
| Irregular | 8 (100) | 9 (81.8) | 5 (83.3) | 1 (50) | 1 (33.3) | 4 (66.7) | |
| Mass margin | ns | ||||||
| Microlobulated | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) | 1 (16.7) | |
| Indistinct | 0 (0) | 3 (27.3) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | |
| Spiculated | 8 (100) | 8 (72.7) | 5 (83.3) | 2 (100) | 3 (100) | 3 (50) | |
| Density | ns | ||||||
| High density | 6 (75) | 10 (90.9) | 5 (83.3) | 2 (100) | 3 (100) | 4 (66.7) | |
| Isodensity | 2 (25) | 1 (9.1) | 1 (16.7) | 0 (0) | 0 (0) | 2 (33.3) | |
| Microcalcification | ns | ||||||
| No | 0 (0) | 5 (45.5) | 2 (33.3) | 1 (50) | 1 (33.3) | 1 (16.7) | |
| Yes | 8 (100) | 6 (54.5) | 4 (66.7) | 1 (50) | 2 (66.7) | 5 (83.3) | |
| Calcification morphology | ns | ||||||
| Punctate | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |
| Amorphous | 3 (60) | 6 (75) | 2 (66.7) | 0 (0) | 2 (100) | 3 (100) | |
| Pleomorphic | 2 (40) | 2 (25) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | |
| Distribution of microcalcifications | ns | ||||||
| Regional | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | |
| Grouped | 4 (80) | 4 850) | 3 (100) | 1 (100) | 1 (50) | 3 (100) | |
| Linear | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Segmental | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Architectural distortion | ns | ||||||
| No | 1 (12.5) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | |
| Yes | 7 (87.5) | 10 (90.9) | 6 (100) | 2 (100) | 3 (100) | 4 (66.7) | |
| Intramammary lymph nodes | ns | ||||||
| No | 6 (75) | 6 (54.5) | 4 (66.7) | 2 (100) | 3 (100) | 6 (100) | |
| Yes | 2 (25) | 5 (45.5) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) | |
| Skin changes | 0.011 | ||||||
| No | 5 (62.5) | 9 (81.8) | 6 (100) | 2 (100) | 0 (0) | 6 (100) | |
| Yes | 3 (37.5) | 2 (18.2) | 0 (0) | 0 (0) | 3 (100) | 0 (0) | |
| Nipple retraction | 0.016 | ||||||
| No | 5 (62.5) | 8 (72.7) | 6 (100) | 2 (100) | 0 (0) | 6 (100) | |
| Yes | 3 (37.5) | 3 (27.3) | 0 (0) | 0 (0) | 3 (100) | 0 (0) | |
5. Discussion
DM is the modality of choice for screening and diagnosis of breast cancer. Since a malignant lesion can be observed in a single section, and the contours of normal breast tissue overlap with the lesion, mammographic sensitivity and tumor visibility may decrease due to poor visualization in dense breast tissue, with a false negativity rate of 8 to 66% (9). Therefore, integration of DBT into DM can increase the radiologists’ confidence, especially in dense breasts, and help detect the lesion borders more accurately (10).
It is important to determine morphological features, such as tumor density, margin, shape, and microcalcification for distinguishing benign lesions from malignant ones (11). The present study, which investigated the superior features of DBT to DM, revealed that DBT visualized the spiculated margins and high-density features of malignant masses more clearly. Moreover, DBT displayed structural distortions, microcalcifications, and foci more accurately than DM. In this regard, Rangarajan et al. reported that DBT is useful in the detection of architectural distortions, visualizing the lesion margins clearly by eliminating tissue overlaps (12).
Although detection of malignant calcifications associated with a small tumor size (barely visible lesions) facilitates mammographic diagnosis, it is very difficult to detect these lesions without calcifications on DM, especially in dense breasts. Evidence suggests that almost 23% of these barely visible malignant lesions are overlooked in DM (13). In another study, DM was insufficient in showing foci and staging compared to DBT, and more lesions were found in 10% of patients by integrating DBT into DM compared to DM alone (14). Therefore, DBT can be a useful modality for mass definition and microcalcification detection in dense breasts, as seen in the majority of our patients.
Molecular classification is important for the prediction of prognosis and effective treatment of breast cancer. The survival rates and treatment options for breast cancer vary depending on subtypes (15). With the detection of various hormone receptors, Ki-67 index, and histological subtypes, aggressiveness of a tumor can be estimated (6). A previous study explained that patients with HER2-enriched tumors have higher rates of nodal involvement, multifocality, intraductal components, and lymphovascular invasion compared to those with luminal A tumors (16).
Recent evidence suggests that radiological appearance may be associated with the molecular subtype and help identify the biological behavior of breast cancer (17, 18). Previous research has investigated the relationship between DM and pathological subtypes (19). However, few studies have investigated the relationship between DBT and pathological subtypes. In this regard, Sartor et al. reported a higher mammographic density in ER-negative tumors, which indicated a poor prognosis compared to other subtypes in a study using DM (20). In our study using DBT, a significant association was found between the tumor grade and density. It is known that malignant masses have higher densities, which may indicate high-grade breast cancer.
Liu et al. revealed a significant relationship between the luminal A subtype and spiculated margins of tumors on DM. They also reported that a lower Ki-67 index and HER2 negativity might be the most important contributors to a spiculated mass (18). Similar to this study, the relationships between morphological features and receptor status, tumor grade, and pathological subgroups on DBT, which is superior to DM in terms of mass definition, were examined in the current study. It was found that spiculated contours were common in ER- and PR-positive groups, and the masses were generally irregular in PR-positive patients.
In line with previous findings (18), ER and PR positivity and HER2 negativity could cause spiculated contours in a mass in the present study. Besides, skin changes and nipple retraction were higher in PR-negative tumors compared to PR-positive tumors. Overall, previous studies have reported significant differences between morphological features and subtypes on DBT. The findings show that DBT can demonstrate the lesion margins, microcalcifications, and lymph nodes more accurately. Besides, the HER2 overexpression subtype was associated with a larger tumor size and more microcalcifications compared to the luminal B subtype on DBT (21). Conversely, a recent prospective population-based study demonstrated no significant difference between DM and DBT in terms of histological subtypes (22). In our study, the frequency of skin changes and nipple retraction was significantly higher in triple-negative tumors compared to other subtypes; in triple-negative tumors with a poor prognosis, this may be an indicator of tumor aggressiveness.
In the present study, regarding the relationship between molecular subtypes and DBT findings, the majority of patients had dense breasts, and significant differences were found between PR-positive and PR-negative dense breasts in terms of mass shape, nipple retraction, and skin changes. While the majority of PR-positive masses in dense breasts had an irregular shape, most of the lesions were round-shaped, which is frequent in PR-negative tumors. On the other hand, in PR-negative tumors, skin changes and nipple retraction were more frequent; there was no significant difference in non-dense breasts. Therefore, DBT can be a useful tool for predicting the PR receptor status of masses, especially in dense breasts.
There were some limitations to our study. First, it was conducted on a relatively small sample size. Second, during the evaluations, interobserver variability could not be assessed, because the two radiologists reached a common consensus.
In conclusion, DBT was found to be superior to DM, as it could visualize the lesion margins, mass density, and architectural distortions more accurately. The majority of PR-positive tumors were irregular, while the majority of PR-negative tumors were round-shaped. Besides, the mass density increased as the tumor grade advanced. Skin changes and nipple retraction were more common in triple-negative tumors compared to other subtypes. Therefore, DBT may be used as a potential diagnostic tool that can show molecular subtypes in dense breasts. Further comprehensive studies are needed to reveal the morphological features of breast cancer subgroups.