1. Background
Thyroid nodules are common findings in clinical practice, with an incidence of 20 - 67%. They are defined as isolated lesions of the thyroid gland, which are radiologically different from the surrounding thyroid tissue (1-3). Evidence shows that the prevalence of thyroid nodules increases with age (4). However, most thyroid nodules are clinically insignificant. They are diagnosed incidentally during neck imaging for other reasons and can be safely managed in surveillance programs (5).
The management of thyroid nodules is based on the nodule function, local compression symptoms, cosmetic complaints, patient anxiety, and risk of malignancy (6-8). According to previous studies, even solid thyroid nodules diagnosed as benign lesions in fine needle aspiration (FNA) may be misdiagnosed as malignant lesions in surgical pathology in 6% of cases (9). Accordingly, various treatments have been suggested for suspected thyroid nodules. So far, several invasive approaches have been proposed for the removal of thyroid nodules. However, these surgical approaches are costly and associated with permanent scars and complications, such as recurrent laryngeal nerve injury, hypocalcemia, hypoparathyroidism, and hemorrhage (10, 11). On the other hand, there are some alternative treatments for benign thyroid nodules, including levothyroxine (LT4) therapy, as well as minimally invasive treatments, such as ethanol injection and radiofrequency (RF) ablation (12, 13).
2. Objectives
This clinical trial aimed to evaluate the management of benign thyroid nodules with ethanol injection, RF ablation, and LT4 therapy.
3. Patients and Methods
3.1. Study Population
This single-blind randomized clinical trial was conducted on 91 patients with benign thyroid nodules and cosmetic complaints, who met the inclusion criteria and presented to different medical centers in Tehran, Iran. Considering the study design, the radiologist who assessed the size of nodules at one, three, and six months after the intervention was blinded to the study. The study protocol was approved by the Medical Ethics Committee of Tehran University of Medical Sciences, and all the patients provided an informed written consent form before participation in the study.
The inclusion criteria of the present study were as follows: (1) willingness to participate in the study; (2) written consent by the patient or family members; (3) age range of 18 - 65 years; (4) normal thyroid tests, including thyroid stimulating hormone (TSH), thyroxine (T4), triiodothyronine (T3), anti-thyroid peroxidase (anti-TPO), and T3 resin uptake (T3RU); (5) solid thyroid nodules and benign solid thyroid nodules, confirmed by ultrasound-guided FNA, as well as a repeat FNA for only benign nodules receiving RF ablation, according to the European Thyroid Association guidelines (2020) (14); (6) normal serum calcitonin levels (serum calcitonin is a marker for diagnosing or confirming the recurrence of medullary thyroid carcinoma).
On the other hand, the exclusion criteria were as follows: (1) patients with malignancy findings; (2) patients with malignant or suspicious pathology findings after ultrasound-guided biopsy; (3) pregnancy; (4) abnormal thyroid tests or serum calcitonin levels; (5) history of treatments, including RF ablation, hormonal therapy, or ethanol injection therapy for thyroid nodules.
3.2. Data Collection Tools
The patients completed a questionnaire, including the demographic information, age, sex, weight, height, smoking habits, family history of thyroid cancer, history of cardiac diseases and osteoporosis, and menstruation status (for females). Thyroid tests, including TSH, T4, T3, anti-TPO, T3RU, and serum calcitonin levels, were recorded at baseline and within one-, three-, and six-month intervals. The levels of TSH (normal range, 0.2 - 4.0 mIU/mL) were determined using an accessible immunoradiometric assay (Sorin Biomedica, Saluggia, Italy). Moreover, the serum levels of total T3 (normal range, 100 - 200 ng/mL), T4 (normal range, 4.6 - 12 ug/dL), anti-TPO antibodies (normal range, 0.0 - 70.0 IU/mL), and calcitonin (normal range < 10 ng/mL) were determined using the radioimmunoassay kits (Radim, Pomezia, Italy).
The nodule volume was assessed by a radiologist using sonography at baseline and within one-, three-, and six-month intervals. Based on the ultrasonic measurements of nodules, three semi-axes were measured, and the nodule volume was calculated with an ellipsoid volume calculator (ellipsoid formula: V = 4.3 × π × Anteroposterior diameter × Transverse diameter × Non-axial view diameter) in SPSS version 25.
3.3. Randomization and Intervention
After explaining the study objectives to the participants, they were asked to provide an informed consent form. Patients who met the inclusion criteria were selected and randomly allocated into three groups: group 1, a single session of RF ablation (n = 31); group 2, a single session of ethanol injection (n = 30); and group 3, a six-month LT4 treatment (n = 30). The patients were allocated to one of the groups through block randomization (groups of six).
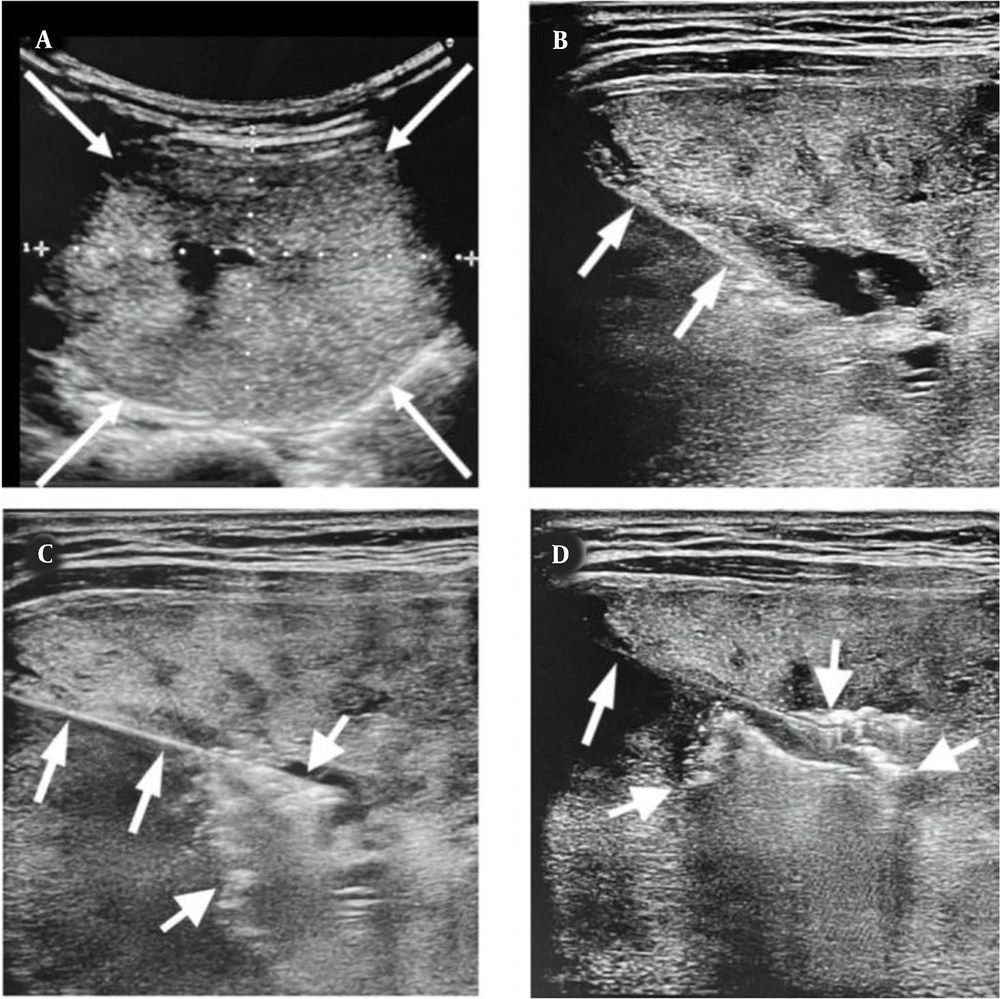
In the RF ablation group, patients with benign thyroid nodules, confirmed by two FNAs, received single-session ablation by an interventionist under ultrasound guidance. After local anesthesia, nodule ablation was performed using an RF generator (Mygen M-2004, 400 KHz, RF Medical Co. Ltd., Korea) with an internally cooled electrode and an active 1-cm tip. The ablation current started from 50 W and elevated at 10 W/min until the emergence of a transient echogenic area on concurrent ultrasounds (Figure 1).
A, The ultrasound shows a large benign thyroid nodule in the right lobe (thin arrows). B, Transverse ultrasound shows the insertion of an RF probe (short arrows) into the middle of the nodule. C, After one minute of ablation, air bubbles (thick arrows) appear in the central part of the nodule and D, Increase after three minutes (thick arrows).
During ethanol injection, sterile ethanol 96% was injected to approximately 30% of the nodule volume (up to 1 mL), using a 22-gauge needle under ultrasound guidance while the patient was in the supine position with a mild neck extension. As mentioned earlier, all injected nodules were solid, and no side effects were observed during injections; however, the patients were allowed to use analgesics if they experienced pain after the procedure.
A standard dose of LT4 (1.6 - 2.1 µg/kg/day to decrease TSH to a range of 0.1 - 0.4 after six months; Euthyrox, Merck, Germany) was administered to the patients in the LT4 treatment group. Depending on the patient’s TSH level, the dose of LT4 was modified on day 35 of the experiment. LT4 was amplified if TSH levels exceeded 0.30 mIU/mL and reduced for patients with unnoticeable TSH or visible symptoms of hyperthyroidism.
The nodule size and nodule volume were examined in all the patients at baseline and within one-, three-, and six-month intervals after the intervention. The volume of thyroid nodule was estimated using a thyroid nodule calculator, based on the sonographic findings at baseline and in the follow-ups (one, three, and six months after the intervention).
3.4. Data Analysis
Data were gathered and analyzed using IBM SPSS for Windows version 25.0 (2017, IBM Corp., Armonk, NY, USA). Moreover, the normal distribution of variables was examined using Kolmogorov-Smirnov test. The nodule volume was compared between the three groups using ANOVA test, and pairwise comparisons were performed using post-hoc tests. Each group was also compared before and after the intervention using repeated measures ANOVA test. A P-value less than 0.05 was considered statistically significant.
4. Results
A total of 91 patients were enrolled in this study, including 31 patients in the RF ablation group (34%), 30 patients in the ethanol injection group (33%), and 30 patients in the LT4 treatment group (33%). Seventy-nine patients (86.8%) were female, and 12 (13.2%) patients were male. None of the participants had a history of thyroid cancer or radiation exposure, while six women had a history of cardiac diseases (n = 3) and osteoporosis (n = 3). Besides, 10 patients were menopausal at baseline.
In the ethanol injection group, 24 (80%) patients were female, while in the RF ablation and LT4 treatment groups, 27 (87.1%) and 28 (93.3%) patients were female, respectively (P = 0.3). The mean age of the patients was 39.1 ± 9.2 years (range: 23 - 64 years). Also, the mean age of the patients in the ethanol injection, RF ablation, and LT4 treatment groups was 36.1 ± 6.2, 38.9 ± 10.1, and 42.3 ± 9.9 years, respectively (P = 0.03). The mean body mass index (BMI) of the patients was also 30.9 ± 3.5 kg/m2 (range: 20 - 59 kg/m2). The mean BMI of the three groups was not significantly different (P = 0.5).
The percentage of volume reduction was calculated in the first, third, and sixth months after the intervention (volume reduction percentage vs. the baseline volume). The mean volume reduction percentage was significantly higher in patients treated with RF and ethanol injection compared to the LT4 group in the first, third, and sixth months. Moreover, in the first and third months, the mean volume reduction was higher in the RF group compared to the ethanol injection group. In the RF and ethanol injection groups, the mean volume reduction percentage was higher in the six-month follow-up compared to the three- and one-month follow-ups; the mean volume reduction was greater in the third month compared to the first month (Table 1).
| Groups | Mean percentage of volume reduction a | P-value (in successive follow-ups) | ||
|---|---|---|---|---|
| One month | Three months | Six months | ||
| Ethanol injection | 38.8 ± 16.2 | 56.9 ± 16.7 | 73.6 ± 12 | < 0.001; 6 Mo > 3; Mo > 1; Mo |
| RF ablation | 48.8 ± 18.2 | 65.7 ± 13.9 | 80.1 ± 24.7 | < 0.001; 6 Mo > 3; Mo > 1; Mo |
| Levothyroxine therapy | 11.1 ± 10.5 | 7.9 ± 12.1 | 8.7 ± 15.9 | 0.33; 6 Mo = 3; Mo = 1; Mo |
| P-value (for the three groups) | < 0.001; RF>Ethanol>Levothyroxine | < 0.001; RF>Ethanol>Levothyroxine | < 0.001; Ethanol = RF>Levothyroxine | |
The Mean Percentage of Volume Reduction in Nodules in the One-, Three-, and Six-month Follow-ups Compared to the Baseline
The association of age and BMI with the percentage of volume reduction in the three treatment groups was evaluated by Pearson's correlation coefficient test. The correlations were weak in all treatment groups and in all three follow-ups (P < 0.4 for all), except for the percentage of volume reduction in the third month in the LT4 treatment group (Pearson’s coefficient = 0.75; P < 0.001) and the percentage of volume reduction in the sixth month in the LT4 treatment group (Pearson’s coefficient = -0.54; P < 0.046).
On the other hand, comparison of the mean percentage of volume reduction between the three groups was performed in each follow-up for women and men separately. The mean volume reduction in men was more significant in the ethanol injection and RF ablation groups compared to the LT4 treatment group in all three follow-ups; however, the mean volume reduction was not significantly different in the ethanol injection and RF ablation groups in the first and third months. On the other hand, the mean percentage of volume reduction in the six-month follow-up was greater in the RF ablation group compared to the ethanol injection group. Besides, the mean volume reduction in women was significantly greater in the ethanol injection and RF ablation groups compared to the LT4 treatment group in all three follow-ups; nevertheless, the mean volume reduction in the ethanol injection and RF ablation groups was not significantly different in the six-month follow-up. Based on the results, the mean percentage of volume reduction was greater in the RF ablation group compared to the ethanol injection group in the first and third months.
5. Discussion
There are various minimally invasive treatments for benign thyroid nodules, including percutaneous ethanol injection (PEI), RF radiation, and laser ablation. The former was first performed in 1990 for autonomously functioning thyroid nodules. This method results in coagulative necrosis and thrombosis of small vessels, followed by inflammatory changes that cause fibrosis, shrinkage, and volume reduction in the treated zone (13, 15-18).
PEI is the first-line treatment for relapsing thyroid cysts, without requiring local anesthesia. Although PEI is associated with some complications due to ethanol leakage into the adjacent structures, including local pain, dysphonia, and major adverse events (eg, recurrent nerve palsy and necrosis of the larynx and skin), none of these complications were observed in the current study (19). In hyperfunctioning nodules, PEI is associated with a risk of hyperthyroidism relapse and progressive regrowth in 70% of the patients. Besides, PEI is contraindicated for solid thyroid nodules, unless it is the only available treatment (18, 20-23). According to previous studies, PEI for non-functioning nodules was associated with a nodular volume reduction of nearly 50% (24-26). Consistent with previous studies, in the current research, PEI was associated with the mean volume reductions of 38.8%, 56.9%, and 73.6% at one, three, and six months after the intervention, respectively.
The RF ablation method was first performed in 2005. This method induces thermal damage, followed by coagulative necrosis in the treated zone, which leads to nodule shrinkage and fibrosis; it is commonly performed under local anesthesia. Perithyroidal hemorrhage, recurrent nerve damage, nodule rupture, and skin burns are some of the common complications secondary to RF ablation (18, 27). However, the rate of complications is low, and they have been only reported in 3.3% of the patients (28, 29). Also, evidence suggests that most of these complications are reversible and eliminated in long-term follow-ups. According to previous studies, RF ablation decreases the nodule size by about 50% after six months and by nearly 80% within one year (28, 30, 31). Similar to previous studies, in the present research, the mean volume reduction in one-, three-, and six-month follow-ups was 48.8%, 65.7%, and 80.1%, respectively in the RF ablation group. It should be noted that during the follow-ups, no complication was observed in our patients.
Moreover, in a study by Mauri et al., percutaneous laser ablation (PLA) and RF ablation were similarly effective for the treatment of benign thyroid nodules, with similar volume reductions at one, six, and 12 months after the intervention and the same rates of complications (31). In a meta-analysis by Chung et al., RF ablation and PLA were compared for the treatment of thyroid nodules. They reported a mean volume reduction of 77.8% within six months in the RF ablation group versus 49.5% in the PLA group. It seems that RF ablation is superior to PLA in decreasing the volume of benign solid thyroid nodules (32).
Additionally, in a randomized trial by Noe Bennedbæk et al., the effect of PEI therapy versus LT4 treatment on benign solitary solid cold thyroid nodules was investigated. They concluded that PEI with a single small dose of ethanol was superior to LT4 suppressive therapy, but not surgery (33). Besides, in a prospective, randomized clinical trial by Huh et al., the efficacy of additional RF ablation treatment sessions on symptomatic benign thyroid nodules was examined. It was concluded that a single session of RF ablation was effective in improving the cosmetic and symptomatic problems of most patients by reducing the nodule volume (34).
Moreover, in a prospective, semi-experimental study by Sung et al. on single-session treatment of benign cystic thyroid nodules with ethanol injection versus RF ablation, it was found that both PEI and RF ablation were helpful and safe modalities; the mean volume reduction was higher in the PEA group compared to the RF ablation group. Therefore, PEA could be the leading treatment modality for cystic thyroid nodules, with a similar therapeutic efficacy to RF ablation; it is also more cost-effective than RF ablation (35).
In conclusion, to the best of our knowledge, no retrospective semi-experimental study has evaluated and compared ethanol injection, RF ablation, and LT4 therapy for patients with benign thyroid nodules. The present findings showed that among the evaluated treatments, RF ablation was associated with the highest mean volume reduction, while the lowest mean volume reduction was observed in the LT4 treatment group during six months of follow-up.