1. Background
Hepatobiliary disorders are common problems during pregnancy, associated with significant maternal and fetal morbidity and mortality (1). Gallstone disease is a hepatobiliary disorder, which occurs in 3 - 10% of all pregnancies (2, 3). Choledocholithiasis is a serious complication of gallstones, which can lead to cholangitis, gallstone pancreatitis with sepsis, and even fetal and maternal death (4). Treatment options for this disorder are limited, although immediate intervention is essential.
Cholecystectomy and common bile duct (CBD) exploration are associated with the increased risk of fetal loss, particularly in early pregnancy (5). Endoscopic retrograde cholangiopancreatography (ERCP) during pregnancy can be challenging for many reasons. Evidence suggests that the risk of pancreatitis is higher in pregnant patients (16%) compared to non-pregnant patients (5%) (6). Besides, pancreatitis may lead to hypovolemia, fetal hypoxia, and death. Radiation exposure is another risk for the patients. Regardless of the shielding method, radiation usually spreads in the body, potentially resulting in fetal anomalies and preterm labor, particularly when ERCP is performed in the first trimester (7). Electrocautery is not completely safe during pregnancy, as amniotic fluid can conduct electrical current to the fetus (8). Balloon sphincteroplasty further increases the risk of pancreatitis (9).
There are limited studies published on the efficacy and safety of transabdominal ultrasound (US)-guided endoscopic treatment for obstructive jaundice during pregnancy. Many researchers have reported their experiences of choledecholithiasis management using endoscopic biliary stenting, without fluoroscopy or any imaging guidance. In this procedure, stent placement is done only after bile aspiration by endoscopy (indicative of proper endoscopic tip placement inside the bile duct) (9). However, most previous studies on this subject are case reports, and in one case series, only six patients were examined. Although this technique avoids the use of radiation, it does not differentiate CBD from cystic duct cannulation, and the stent may be inserted in the gallbladder (9).
2. Objectives
In this study, we aimed to evaluate the technical success, clinical success, safety, complications, and procedure time in ten pregnant patients with obstructive jaundice, for whom endoscopic biliary stenting was achieved under transabdominal US guidance, without any radiation exposure.
3. Patients and Methods
We analyzed the data of ten pregnant women, presenting with biliary obstruction to Suez Canal University Hospital, Ismailia, Egypt, between January 2018 and October 2020. The extracted data included the cause of referral and laboratory data, including the serum levels of bilirubin, amylase, and lipase, liver enzymes and coagulation profile, and white blood cell count before and 24 hours after the intervention. The data also included the results of hepatobiliary US examination, the diameter of CBD, the number and size of CBD stones, the presence or absence of intrahepatic biliary dilation, and pancreatic size and texture. This study was approved by the local ethics committee and complied with the ethical standards of the national and institutional research committees. A formal written consent form was obtained from all of the studied patients.
The patients were assessed by an obstetrician for fetal wellbeing and by an anesthesiologist in terms of fitness for general anesthesia and endotracheal intubation. The patients fasted for six hours before endoscopy. The procedure was performed under general anesthesia, with propofol used to induce and maintain anesthesia. Endotracheal intubation was also performed for all patients to avoid aspiration; the patients were kept in a left lateral position during the procedure. A single gastroenterologist (MMA), with 23 years of experience in endoscopy, performed the procedures.
The endoscope (PENTAX ED-3-I10T, Tokyo, Japan) was advanced into the duodenum, and the papilla was localized and cannulated with a standard hydrophilic guide wire (280 cm, 0.035''; Cook Medical Inc., Winston-Salem, USA). After CBD cannulation, a radiologist (MAA), with 18 years of experience in US examinations, performed an abdominal US examination to confirm deep biliary cannulation and the wire position inside the CBD. Besides, a minor papillotomy was performed for one patient to facilitate stent insertion (Sphincterotome, Cook Medical, Winston-Salem, USA). No contrast was injected in this study.
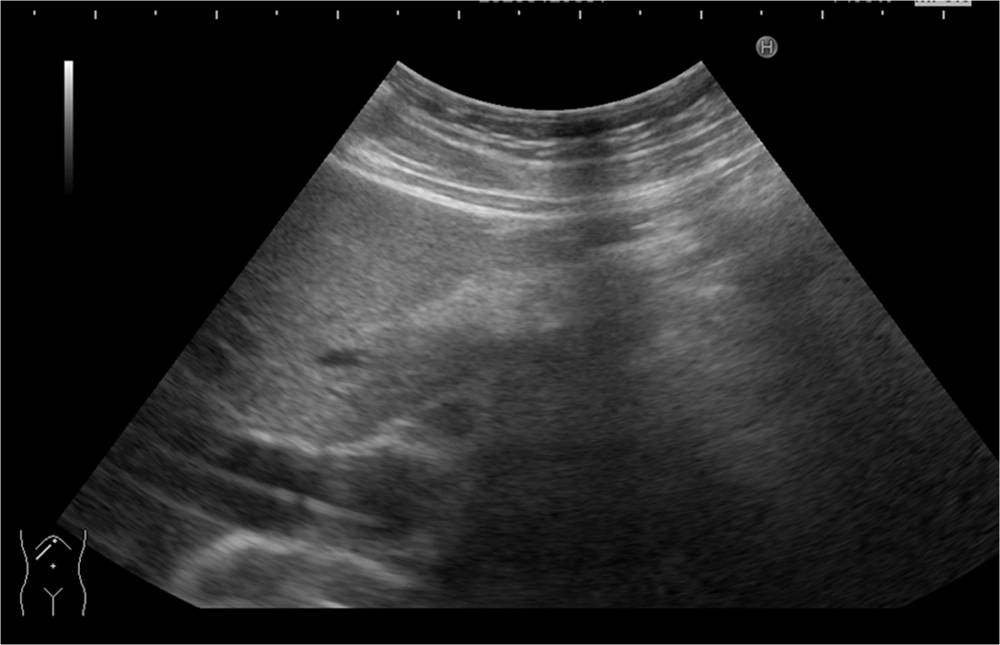
After confirming deep biliary cannulation, a sheath was introduced into the CBD. In case of non-visualization of the wire by US, another trial of wire manipulation was carried out to ensure the proper wire position inside the CBD. Another US scan was performed before advancement of the stent to ensure that the wire and the sheath were in the correct positions (Figure 1). In this study, plastic stents (10-French, 10 cm; Cook Medical, Winston-Salem, USA) were used. The stent was then advanced, and the free bile flow was confirmed. We tried to achieve biliary decompression using the least invasive technique. The procedure time was calculated from the onset of anesthesia induction until the start of recovery.
The patients were followed-up and discharged as per protocol. The follow-up serum amylase and lipase levels were measured at six and 24 hours after the procedure. Adverse endoscopic outcomes, including pancreatitis, fever, cholangitis, or evidence of fetal compromise, were assessed based on standard definitions. Fetal complications were also assessed after endoscopic biliary stenting, before discharge, at delivery, and one month after delivery. All patients were followed-up until delivery.
4. Results
This study was conducted in the endoscopy unit of Suez Canal University Hospital in Ismailia, Egypt. This is a tertiary care referral center, covering five Egyptian governorates and a large number of patients per year. Ten pregnant women, who underwent endoscopic biliary stenting between January 2018 and October 2020, were included in this study. For every patient, comprehensive medical history-taking and clinical examination were performed. The patients’ age ranged from 19 to 33 years (mean: 23 years). Six patients were in the first trimester, three patients in the second trimester, and one patient in the third trimester. The causes of referral included biliary colic (n = 6), gallstone pancreatitis (n = 1), and jaundice with elevated liver enzymes (n = 3).
The patients were admitted to the internal medicine department, where routine laboratory tests, including complete blood count (CBC), coagulation profile, serum amylase, lipase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total and direct serum bilirubin, were performed. The total serum bilirubin ranged from 2.7 to 7.9 mg/dL (mean: 4.2 mg/dL); the direct serum bilirubin level ranged from 2 to 5.5 mg/dL (mean: 2.9 mg/dL); the ALT level ranged from 109 to 1166 U/L (mean: 215 U/L); the AST level ranged from 92 to 907 U/L (mean: 119 U/L); and the serum amylase level ranged from 23 to 2012 U/L (mean: 83 U/L) (Table 1).
| Variables | Values (mean) |
|---|---|
| Maternal age (y) | 19 - 33 (23) |
| Gestational age (weeks) | 5 - 33 (12) |
| Cause of biliary obstruction | |
| Stone | 10 |
| Mass | 0 |
| Clinical presentations | |
| Jaundice | 10 |
| Biliary colic | 10 |
| Fever | 0 |
| Laboratory data | |
| Total bilirubin (mg/dL) | 2.7 - 7.9 (4.2) |
| Direct bilirubin (mg/dL) | 2 - 5.5 (29) |
| AST (U/L) | 92 - 907 (119) |
| ALT (U/L) | 109 - 1166 (215) |
| Amylase (U/L) | 25 - 2012 (83) |
| Lipase (U/L) | 5 - 210 (99) |
| Ultrasound findings | |
| Single CBD stone | 8 |
| Multiple CBD stones | 2 |
| Caliber CBD (mm) | 9 - 18 (13) |
| Bulky pancreas | 1 |
| Peripancreatic fluid | 1 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Moreover, a focused hepatobiliary US examination was performed for the patients. CBD stones were evident in seven patients. Two patients had multiple CBD stones. The maximum stone diameter ranged from 5 to 14 mm. All patients had dilated CBD, with the diameter ranging from 9 to 18 mm. One patient showed bulky pancreas and a rim of peripancreatic fluid collection. Selective cannulation of CBD was successful in all cases and confirmed by an abdominal US scan of CBD to visualize the wire. The free bile flow was immediately achieved; however, one patient required mild sphincterotomy. The procedure time ranged from eight to 14 minutes (mean: 10 min).
No cases of postoperative pancreatitis were encountered in this study. Biliary colic markedly improved in all patients, with no need for analgesia after stenting. All patients were discharged from the hospital at one day post-stenting. The obstetric assessment revealed no fetal or obstetric complications. One patient developed cholangitis at three months post-stenting, and re-stenting was carried out in the same manner. Another patient developed acute cholecystitis at ten days post-stenting and was medically treated (Table 2).
| Outcomes | Values (mean) |
|---|---|
| Successful papilla cannulation | 10/10 |
| Successful wire placement in the CBD | 10/10 |
| Successful stent placement | 10/10 |
| Sphincterotomy | 1/10 |
| Technical success | 10/10 |
| Procedure time (min) | 8 - 14 (10) |
| Clinical success/resolution of symptoms | 10/10 |
| Postoperative complications | |
| Acute cholecystitis | 1/10 |
| Acute cholangitis | 1/10 |
| Others | 0/10 |
| Obstetric/fetal complications | 0/10 |
Abbreviations: US, ultrasound; CBD, common bile duct.
5. Discussion
Pregnancy is associated with an increased risk of hepatobiliary disorders and possibly with impaired gallbladder emptying during pregnancy (10). Stagnation of bile can cause retention of cholesterol crystals and gallstone formation (10). These stones are typically small in size and can easily migrate to the CBD. However, there is no information about the management of CBD stones during pregnancy.
Generally, ERCP is a risky procedure, with an even higher risk during pregnancy. In most previous studies, the risk of post-ERCP pancreatitis has been estimated at 5%, increasing to about 16% in pregnant women (6). It is well-established that prolonged endoscopic manipulation, sphincterotomy, percutaneous sphincterotomy, and pancreatic contrast injection are risk factors for post-ERCP pancreatitis (11). Also, radiation exposure during fluoroscopy is of particular importance due to its teratogenic and carcinogenic effects, especially in the first trimester of pregnancy (12).
The application of US-guided endoscopic biliary stenting has been only recently reported. In this regard, Sharma and Maharshi described a two-step procedure, using biliary sphincterotomy and stenting without fluoroscopy (13). In another study, selective deep cannulation was confirmed by bile aspiration; the bile appearance was used as a guide for CBD cannulation (14). However, bile aspiration could not differentiate between biliary and cystic duct cannulation, and the stent might be inserted into the gallbladder.
In the present study, ten pregnant patients in various trimesters underwent stenting under transabdominal US guidance, without contrast injection or fluoroscopy. All patients had uneventful pregnancies with normal fetal outcomes; neonatal health and birth weight were normal at full term in all cases. In another study of 68 ERCP cases in 65 pregnancies, 11 (16%) cases of pancreatitis were reported (15). The fetal outcome was worse in patients undergoing ERCP in the first trimester (15); however, 53 (90%) patients achieved a full-term pregnancy (15, 16). Only 73% of mothers who underwent ERCP in the first trimester delivered at term (16); low birth weight was reported in 21% of cases (16). In another study of 20 pregnant patients undergoing ERCP, one neonatal death was encountered at 26 hours after birth; she underwent three ERCPs during pregnancy with stenting of the pancreatic duct in each procedure (17). Another patient had an inexplicable spontaneous abortion at three weeks post-ERCP (17).
Moreover, in a study on 58 pregnant patients undergoing ERCP, the risk of post-ERCP pancreatitis was estimated at 12%; the increased rate as compared to non-pregnant patients (5%) was attributed to the limited use of fluoroscopy for guiding the wire during deep biliary cannulation (18). In the present study, no cases of post-endoscopic biliary stenting pancreatitis were reported; this might be attributed to the lack of contrast injection or wide sphincterotomy, which are definite predisposing factors for pancreatitis. Besides, in our study, no cases of abortion or preterm delivery were documented, which might be related to the absence of fluoroscopy with its teratogenic effects and the shorter duration of the procedure, which can decrease the dose of anesthetics needed.
All of our patients underwent another endoscopic biliary stenting at two months after delivery. The stent was removed, and CBD sweeping was carried out with a balloon after complete sphincterotomy, as the stent softens the stones. We recommend US-guided endoscopic biliary stenting and decompression during pregnancy and postponing the subsequent clearance of CBD until after delivery. In our study, the procedure time was short and comparable to fluoroscopy and contrast injection. The US guidance did not lead to an increase in the procedure time, which was 8 - 14 minutes (mean: 10 min); this was expected as the lack of contrast injection and fluoroscopic evaluation was compensated for by the US assessment.
There are several limitations in this study. First, the number of patients included was limited. Second, we were unable to clear CBD stones using US without fluoroscopy. Therefore, this option was not offered to the patients, as we felt that it would be challenging to provide; also, it was not one of the study objectives. Third, this study was conducted by a single radiologist and endoscopist; therefore, it was not possible to assess the feasibility of the procedure at different levels of experience. Fourth, we did not compare the aspiration technique and US guidance, as it required a large number of patients. Finally, no cases of malignant obstructive jaundice or benign strictures were encountered; they might have affected the level of cannulation difficulty and the success rate of US guidance.
In conclusion, abdominal US-guided endoscopic biliary stenting, without fluoroscopy or contrast injection, is an effective and safe method for managing pregnant women with calcular obstructive jaundice.