1. Background
Urolithiasis is a lifetime disease with a prevalence of 10% - 15% and a recurrence rate of up to 50% within five years (1, 2). Urolithiasis may lead to several complications, such as urinary tract obstruction, urinary tract infections, and chronic renal impairment (3, 4). Generally, the management and follow-up of urinary calculi depend on the size, location, and composition of stones, as well as the anomalies of the urinary tract. Analysis of stone composition is usually done by infrared spectroscopy (IRS) or X-ray diffraction after stone extraction (5, 6).
Pretreatment identification of the stone composition influences the treatment plan and stone recurrence preventive regimen. For example, uric acid (UA) stones may be treated by alkalization of urine. However, for calcium and struvite stones, more invasive medical approaches, such as extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy, are needed (7, 8). Overall, accurate identification of stone composition is only possible after extraction. Therefore, it is necessary to find a non-invasive and simple tool to predict the stone composition in vivo.
Dual-energy computed tomography (DECT) has been used to discriminate the urinary stone composition (9). Several DECT scanners, including dual-source dual-energy computed tomography (DSDECT), rapid kV-switching single-source DECT, and single-source dual-layer DECT, are used to perform dual-energy examinations. Stones can be classified into pure and mixed stones using post-processing material decomposition techniques. To date, most studies have reported that DECT can distinguish pure stones with high accuracy (8, 10-17). While stones are commonly composed of a mixture of substances, only few studies have focused on these compositions (18).
2. Objectives
The current study aimed to evaluate the diagnostic accuracy of DSDECT in predicting the components of mixed urinary stones by using postoperative IRS in vitro as the standard method.
3. Patients and Methods
3.1. Patient Population
This retrospective review was performed among patients undergoing surgical treatment of urinary tract calculi, which were examined based on the DSDECT protocol before surgery and diagnosed by IRS postoperatively. The patients underwent endoscopic or laparoscopic surgical treatment at the First Affiliated Hospital of Nanjing Medical University (Jiangsu, China) between June 2018 and March 2020. A total of 117 mixed urinary stones, detected by IRS in 111 patients, were retrieved from the medical files archived in the Department of Urology. Patients with a pure stone composition diagnosed by IRS were excluded from the study.
3.2. DSDECT Protocol and Image Analysis
A third-generation DSDECT system (Somatom Force, Siemens Healthcare, Germany) was used for unenhanced CT scan. The scan parameters were as follows: Tin filtration with Sn100 kV and Sn150 kV; quality reference mAs: 250 and 120 mAs; 32 mm × 0.6 mm collimation; rotation time of 0.6 seconds; and pitch of 0.6. A real-time automatic tube current modulation system (CARE Dose4D; Siemens) was used for this purpose. Also, the reconstruction slice thickness was 0.6 mm with a reconstruction increment of 0.4 mm. Three series of images (Sn100 kV, Sn150 kV, and an average-weighted image by fusing the Sn100 kV and Sn150 kV images with a weighting factor of 0.5) were reconstructed from the DECT data.
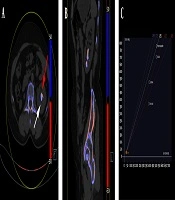
The images were sent to a postprocessing workstation (Syngo Dual Energy, version VB10B; Siemens Healthcare, Germany), and the stone components were predicted by the “Kidney Stones” software application, which is based on different absorptions of X-ray with different energies. According to the method described in the literature, the chemical composition of urinary stones was classified into four types, namely, calcium oxalate (CaOx), hydroxyapatite (HA), cystine (CYS), and UA (16). This application attributes different colored overlays to different calculi. Figure 1 presents a detailed stepwise analysis. Two experienced uroradiologists assessed all of the images in consensus.
3.3. In Vitro IRS Analysis
A postoperative IRS analysis was performed (LIIR-20, Lambda Scientific Instrument, Tianjin, China), based on the standard method in vitro. A stone was defined as mixed when its minor components constituted > 15% of the stone (8, 19). IRS can differentiate more types of chemical components than the “Kidney Stones” application. CaOx monohydrate and CaOx dihydrate were classified as CaOx, and ammonium urate and anhydrous UA as UA to simplify the interpretation of the analytical results of chemical composition by IRS. Meanwhile, carbapatite stones and magnesium ammonium phosphate were classified as HA for a similar anti-infective management (due to software limitations).
3.4. Statistical Analysis
Statistical analysis was performed in SPSS version 20 (IBM Inc., Chicago, IL, USA). The patients’ general characteristics were recorded in this study. Continuous variables are presented as mean ± standard deviation (SD). For the same mixed stones, a paired t-test was performed to compare different CT values under 100 kV and 150 kV. Analysis of variance (ANOVA) test was used to compare differences in the ratio and CT values. Categorical variables are presented as frequency or percentage. The stone components according to DSDECT were compared with the IRS results based on the diagnostic performance study (accuracy, sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]).
4. Results
From June 2018 to March 2020, 111 consecutive patients with 117 mixed urinary stones, detected by IRS postoperatively, were included in this study. All 111 patients with suspected urolithiasis underwent preoperative DSDECT of the abdomen and pelvis preoperatively. The patients included 73 male and 38 female patients with a mean age of 49.3 ± 12.8 years (range: 20 - 81 years), a mean body mass index (BMI) of 24.8 ± 3.2 kg/m2 (range: 17.7-35.1 kg/m2), and a mean stone size of 18.5 ± 12.1 mm (range: 6.0 - 70.0 mm). All patients were subsequently treated with surgical procedures, and the IRS stone analysis was performed in vitro postoperatively.
Of 111 urolithiasis cases, one patient had two stones, one in the kidney and one in the bladder; one patient had two stones, one in each ureter; two patients had two stones, one in the ureter and one in the kidney; and two patients had two stones, one in each kidney. Among 117 urinary stones, 75 were kidney stones, 38 were ureter stones, and four were bladder stones. According to the stone composition characterized by IRS, 117 mixed urinary calculi, which were composed of a main constituent and minor admixtures, were divided into four groups: CaOx mixed with HA (n = 70), HA mixed with CaOx (n = 36) (Figure 2), UA mixed with CaOx (n = 8), and CYS mixed with HA (n = 3). The clinical characteristics of the four groups are summarized in Table 1.
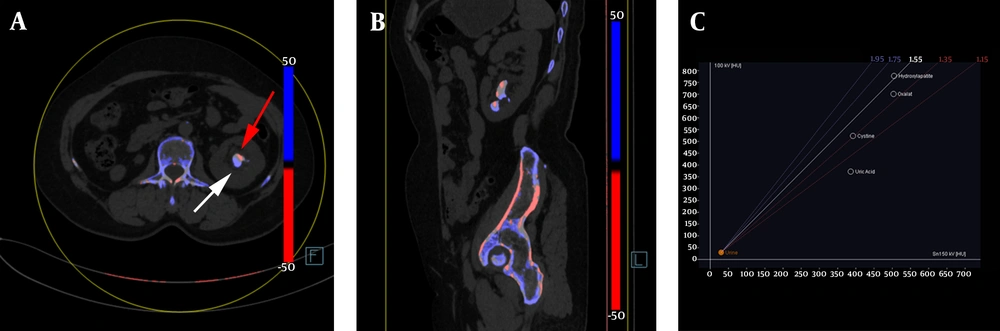
The color-coded dual-source dual-energy computed tomography (DSDECT) image showing an example of mixed renal hydroxyapatite (HA)-calcium oxalate (CaOx) stone. A, The HA part is color-coded in blue (white arrow), and the CaOx part is color-coded in red (red arrow) in the axial image of mixed stone; B, Sagittal image of the mixed stone; C, The setting dialog of DSDECT with a ratio value of 1.55.
| IRS composition | Gender | Age, y | BMI, kg/m2 | Size, mm | Site | Surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Kidney | Ureter | Bladder | PCNL | URL | Lithotriptoscopy | Laparoscopic | ||||
| CaOx-HA | 48 | 19 | 49.3 ± 13.0 | 25.0 ± 3.2 | 17.6 ± 9.9 | 44 | 25 | 1 | 28 | 40 | 1 | 1 |
| HA-CaOx | 20 | 14 | 49.2 ± 10.3 | 24.7 ± 3.3 | 16.2 ± 12.0 | 21 | 12 | 3 | 16 | 17 | 3 | / |
| UA-CaOx | 5 | 3 | 59.0 ± 6.1 | 24.3 ± 2.0 | 31.9 ± 19.2 | 7 | 1 | / | 6 | 2 | / | / |
| CYS-HA | 1 | 2 | 21.7 ± 2.1 | 21.4 ± 3.4 | 29.3 ± 16.9 | 3 | / | / | 3 | / | / | / |
| Total | 73 | 38 | 49.3 ± 12.8 | 24.8 ± 3.2 | 18.5 ± 12.1 | 75 | 38 | 4 | 53 | 59 | 4 | 1 |
Abbreviations: BMI, body mass index; CaOx-HA, calcium oxalate mixed with hydroxyapatite; CYS-HA, Cystine mixed with hydroxyapatite; HA-CaOx, hydroxyapatite mixed with calcium oxalate; IRS, infrared spectroscopy; PCNL: percutaneous nephrolithotomy; UA-CaOx, uric acid mixed with calcium oxalate; URL, ureterorenoscopy lithotripsy.
The predicted compositions by DSDECT for all 117 mixed stones are summarized in Table 2. According to DSDECT, seven types of stones, namely, CaOx-HA, HA-CaOx, UA-CaOx, CYS-HA, UA-HA, CYS-CaOx, and pure HA stones, were found. DSDECT had an overall accuracy of 51.3% (60/117) for predicting different compositions of 117 mixed urinary calculi compared to the IRS as the reference standard. The accuracy of DSDECT for detecting all components of CaOx-HA, HA-CaOx, UA-CaOx, and CYS-HA was 68.4% (80/117), 64.1% (75/117), 97.4% (114/117), and 99.1% (116/117), respectively. Table 3 summarizes the diagnostic parameters for predicting different components of each group.
| DSDECT | IRS composition | Total | |||
|---|---|---|---|---|---|
| CaOx-HA | HA-CaOx | UA-CaOx | CYS-HA | ||
| CaOx-HA | 33 | / | / | / | 33 |
| HA-CaOx | 26 | 20 | / | / | 46 |
| UA-CaOx | / | / | 5 | / | 5 |
| CYS-HA | / | / | / | 2 | 2 |
| UA-HA | / | / | 3 | / | 3 |
| CYS-CaOx | / | / | / | 1 | 1 |
| HA | 11 | 16 | / | / | 27 |
| Total | 70 | 36 | 8 | 3 | 117 |
Abbreviations: CaOx-HA, calcium oxalate mixed with hydroxyapatite; CYS-CaOx, cystine mixed with calcium oxalate; CYS-HA, cystine mixed with hydroxyapatite; DSDECT, dual-source dual-energy computed tomography; HA, hydroxyapatite; HA-CaOx, hydroxyapatite mixed with calcium oxalate; IRS: infrared spectroscopy; UA-CaOx, uric acid mixed with calcium oxalate; UA-HA, Uric acid mixed with hydroxyapatite.
| ACC, % | 95% CI, % | SEN, % | 95% CI, % | SPE, % | 95% CI, % | PPV, % | 95% CI, % | NPV, % | 95% CI, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| CaOx-HA | 68.4 | 68.0 - 68.7 | 47.1 | 35.4 - 58.8 | 100 | 100 - 100 | 100 | 100 - 100 | 56.0 | 45.3 - 66.6 |
| HA-CaOx | 64.1 | 63.7 - 64.5 | 55.6 | 39.3 - 71.8 | 67.9 | 57.7 - 78.1 | 43.5 | 29.2 - 57.8 | 77.5 | 67.7 - 87.2 |
| UA-CaOx | 97.4 | 97.4 - 97.5 | 62.5 | 29.0 - 96.0 | 100 | 100 - 100 | 100 | 100 - 100 | 97.3 | 94.3 - 100 |
| CYS-HA | 99.1 | 99.1 - 99.2 | 66.7 | 13.3 - 100 | 100 | 100 - 100 | 100 | 100 - 100 | 99.1 | 97.4 - 100 |
Abbreviations: ACC, accuracy; CaOx-HA, calcium oxalate mixed with hydroxyapatite; CI, confidence interval; CYS-HA, cystine mixed with hydroxyapatite; DSDECT, dual-source dual-energy computed tomography; HA-CaOx, hydroxyapatite mixed with calcium oxalate; NPV, negative predictive value; PPV, positive predictive value; SEN, sensitivity; SPE, specificity; UA-CaOx, uric acid mixed with calcium oxalate.
The imaging characteristics of different mixed urinary stones scanned by DSDECT showed that the CaOx-HA ratio value was lower than that of HA-CaOx (1.59 ± 0.11 vs. 1.66 ± 0.22; P < 0.05); however, both were higher than the ratio values of UA-CaOx and CYS-HA. Moreover, the 150-kV CT values of CaOx-HA were higher than those of the other three groups, while they were lower than those of CaOx-HA (915.41 ± 226.84 vs. 1424.93 ± 340.59; P < 0.01) (Table 4).
| IRS composition | n | Ratio | CT values (HU), 100 kV | CT values (HU), 150 kV |
|---|---|---|---|---|
| CaOx-HA | 70 | 1.59 ± 0.11 | 1424.93 ± 340.59 | 915.41 ± 226.84AA |
| HA-CaOx | 36 | 1.66 ± 0.22* | 1284.39 ± 392.16 | 799.56 ± 252.01*AA |
| UA-CaOx | 8 | 1.37 ± 0.15**## | 857.13 ± 354.36**## | 623.38 ± 200.77**AA |
| CYS-HA | 3 | 1.37 ± 0.03*## | 693.00 ± 78.50**## | 529.67 ± 63.89**A |
Abbreviations: CaOx-HA, calcium oxalate mixed with hydroxyapatite; CYS-HA, cystine mixed with hydroxyapatite; DSDECT, dual-source dual-energy computed tomography; HA-CaOx, hydroxyapatite mixed with calcium oxalate; UA-CaOx, uric acid mixed with calcium oxalate.
aComparison of differences in the ratios or CT values (*, P < 0.05 and **, P < 0.01 compared to the CaOx-HA group; #, P < 0.05 and ##, P < 0.01 compared to the HA-CaOx group; A, P < 0.05 and AA, P < 0.01 compared to 100-kV CT values for the same mixed stones.
5. Discussion
Urinary stone differentiation is crucial in treatment planning. However, it is unclear whether preoperative DSDECT and postoperative IRS analysis may be used interchangeably for mixed urinary stones. In the present study, we found that the accuracy of DSDECT for predicting all stone components was 51.3%, which is low. Although this modality showed good accuracy in predicting the components of UA-CaOx and CYS-HA stones in vivo, it had a relatively low accuracy for CaOx-HA and HA-CaOx stones.
DECT is a new technology to better characterize urinary calculi. It is based on acquiring two different datasets at different kV values from the same material; the material component is calculated by attenuation differences at different energy levels (9). Several types of DECT scanners with inherently different acquisition and postprocessing techniques, such as DSDECT, rapid kV-switching single-source DECT, and single-source dual-layer DECT, are commercially available (20). A DSDECT scanner is composed of two tubes that can provide high temporal resolution for characterization of urinary stone composition using an advanced postprocessing program. The use of DSDECT for determining the urinary stone composition has been widely investigated. According to the literature, most studies have focused on pure urinary calculi and reported a good agreement with postoperative IRS stone analysis (12-17), even for patients with a large body habitus (11).
The most successful application of DSDECT is to differentiate UA from non-UA calculi, as shown in in vitro and in vivo studies. Primak et al. (15) found that DSDECT could accurately discriminate UA stones from non-UA stones with 93% accuracy and 94% sensitivity in an anthropomorphic phantom model. In another in vivo study by Habashy et al. (13), they predicted 15 UA stones by DSDECT in individuals, who were later administered dissolution therapy. Twelve of these patients had successful outcomes, which eliminated the need for a surgical intervention and could be useful for urologists.
However, most urinary calculi are mixed, containing two or more compositions (18) that are seldom studied. Therefore, identifying and quantifying the individual components in each stone are essential to ensure proper management. In this regard, Leng et al. (18) used DSDECT to quantify the UA and non-UA compositions of 24 mixed urinary stones in vitro and present an accurate quantification of UA and non-UA components in mixed urinary calculi. However, they did not differentiate the composition of non-UA stones in their study.
Several other studies have used DSDECT to differentiate stone materials, but have only included few mixed stones in addition to pure stones (19, 21-25). Thomas et al. (23) concluded that DSDECT could distinguish between calcified and non-calcified calculi in their assessments using only two mixed stones in vivo. Besides, Stolzmann et al. (25) differentiated UA-containing and non-UA-containing urinary stones in six pure and 29 mixed stones, using a DSDECT scanner and reported sensitivity, specificity, PPV, and NPV of 88.9%, 97.7%, 88.9%, and 97.7%, respectively. On the contrary, Manglaviti et al. (24) reported that DSDECT had a poor agreement with IRS for identifying chemical composition of mixed stones. In their study, there were totally five UA-HA mixed stones determined by IRS, of which four were misclassified as CYS-HA by DSDECT in vivo (24).
All of the mentioned studies on mixed urinary stones focused on the differentiation of UA and non-UA or calcified and non-calcified compositions of stones; however, the researchers did not discriminate other compositions. The present study showed that DSDECT has a high accuracy in predicting the presence of UA-CaOx and CYS-HA in mixed urinary stones (97.4% and 99.1% accuracy, respectively). However, the accuracy of detecting CaOx-HA and HA-CaOx is relatively low (68.4% and 64.1%, respectively). This finding may be related to several overlaps in the color overlay values between CaOx and HA. Also, we evaluated the imaging characteristics of different mixed urinary stones, scanned by DSDECT in vivo, which showed the lower ratio value of CaOx-HA than HA-CaOx. Meanwhile, 150-kV CT values of CaOx-HA were higher than those of the other three groups. Therefore, combination of DSDECT with the measured ratio and CT values may help differentiate CaOx-HA and HA-CaOx stones.
A possible explanation for the observed differences between the two methods may be the use of different DECT machines. Generally, different DECT machines and parameters are used to identify stone compositions. However, DSDECT used in our study was a third-generation scanner with an enhanced graphic processor (26); the parameters used in this study agree with previous studies (27, 28). Another explanation for the observed differences may be the IRS stone analysis. Although IRS has been accepted as the reference standard in the urinary stone analysis in vitro, only part of the calculi was tested, limiting its ability to detect all of the compositions accurately (29).
There are several limitations in the present study. The first limitation is the small size of stones containing UA and CaOx and stones containing CYS and HA. Although the samples of mixed stones in our in vivo study were larger than the literature, the size of CYS-HA stones was small (only three samples), and other samples, such as CaOx-CYS and UA-HA stones, were lacking. The second limitation is that DSDECT can only differentiate stone compositions as CaOx, HA, UA, and CYS. In contrast, IRS can identify more compositions. For example, calcium monohydrate oxalate should be treated differently from calcium dihydrate oxalate, which could not be differentiated by DSDECT (16, 30). Thirdly, only two-component mixed compositions were evaluated in this retrospective study, while no other compositions were included.
In conclusion, although DSDECT can accurately predict all components of UA-CaOx and CYS-HA stones, it has a low accuracy in determining the components of mixed urinary CaOx-HA and HA-CaOx calculi in vivo. Therefore, combination of DSDECT with the measured ratio and CT values may help differentiate CaOx-HA and HA-CaOx stones.