1. Background
Breast cancer is the most common cancer among women worldwide (1, 2). Accurate and early diagnosis and prompt treatment can improve the outcomes of patients with breast cancer. Ductal carcinoma in situ (DCIS) is an early-stage, non-invasive breast cancer. It is characterized by the abnormal proliferation of malignant epithelial cells, confined to the breast ductal lobular system, with no evidence of infiltration through the basement membrane into the surrounding stroma (3, 4). Due to advances in imaging technologies, along with an increasing understanding of the pathology of DCIS, increasing cases of DCIS have been diagnosed in recent years (5-7).
Mammography is a common primary test for breast cancer screening (8). Once a lesion is identified by a mammogram or physical examination, ultrasound examination can be used for further characterization (9, 10). Besides, ultrasound examination may be superior to mammography, which is the primary screening test in women with dense breast tissues (11). The American College of Radiology (ACR) has proposed the Breast Imaging-Reporting and Data System (BI-RADS) to classify breast lesions into six categories (12). Ultrasound examination is important for the BI-RADS classification. Patients diagnosed with BI-RADS 4A lesions or lower categories are generally considered to have a low suspicion for malignancy, whereas patients with BI-RADS 4B lesions or higher categories should undergo further tissue diagnosis to rule out malignancy (13). Accordingly, the BI-RADS classification has become a key process in the diagnosis of breast lesions.
Previous studies have demonstrated the clinical validity of the BI-RADS assessment (14-16). However, even patients with BI-RADS 4A lesions or lower categories can still have DCIS (17-19), which is probably due to the atypical presentations of DCIS (20). The underestimation of DCIS into lower categories via ultrasound BI-RADS assessment may delay early diagnosis and treatment. There is a paucity of data to determine the causes of underestimation of breast lesions based on ultrasound BI-RADS assessment in patients with DCIS.
2. Objectives
This study aimed to investigate the underestimation of DCIS based on ultrasound BI-RADS classification and to explore the related factors.
3. Patients and Methods
3.1. Study Design
In this cross-sectional study, conducted from January 2015 to May 2017, consecutive patients, presenting to the department of breast surgery of our hospital, were screened and retrospectively examined. Patients who met the following criteria were included in the present study: (1) undergoing a breast ultrasound examination, (2) surgical resection for abnormal ultrasound findings within two weeks after ultrasound, (3) DCIS diagnosis according to the postoperative pathology report. On the other hand, patients with incomplete data were excluded. The demographics, clinical information, pathological reports, and ultrasound assessments were collected and analyzed. The study protocol was approved by the institutional ethic committee of our hospital (No.2020-866). Written informed consent was obtained from all patients for research and publication purposes. The patients’ information was kept confidential according to the Declaration of Helsinki.
3.2. Ultrasound Measurements
Breast ultrasound examinations were performed by certified ultrasonographers, using an HI Vision Avius L color ultrasound system (HITACHI, Japan) and a 5 - 13 MHz broadband linear array probe. Breast lesions found in the ultrasound examination were classified into six categories according to the BI-RADS lexicon (12). The ultrasound features of breast lesions included shape (oval, round, or irregular), sonographic pattern (cumulus, coral, pipe, or miscellaneous), margin (circumscribed or not), orientation (parallel to the skin line or not), echo pattern (anechoic, hyperechoic, isoechoic, complex cystic or solid lesion, hypoechoic, or heterogeneous), posterior features (no posterior features, enhancement, shadowing, or combined pattern), architectural distortion (yes or no), microcalcifications (in or outside of a lesion), duct changes (yes or no), and vascularity (absent, internal vascularity, or vessels in rim). All ultrasounds were evaluated independently by two ultrasonographers who had more than 10 years of experience in ultrasound examinations. Any discrepancy among ultrasonographers was discussed until a conclusion could be drawn.
3.3. Pathological Examination of Surgical Specimens
Surgical resection of abnormal breast lesions after ultrasound was performed by senior surgeons in our hospital. Every surgical resection specimen was sent to the laboratory for pathological examination. DCIS with microinvasions was defined according to the World Health Organization (WHO) classification (21). Also, DCIS was diagnosed and classified according to the criteria of Van Nuys classification system (22). Grade 1 was defined as non-high nuclear grade DCIS without necrosis; grade 2 was defined as non-high nuclear grade DCIS with necrosis; and grade 3 was defined as high nuclear grade DCIS with or without necrosis. Underestimation was defined when a lesion classified as category 4A or lower was confirmed as DCIS based on the postoperative pathology report. On the other hand, non-underestimation referred to lesions classified as category 4B or higher.
3.4. Statistical Analysis
Continuous variables with a normal distribution are reported as mean ± SD (standard deviation) and compared by student’s t-test. Non-normally distributed variables are expressed as median (interquartile range [IQR]) and compared using Wilcoxon two-sample test. Categorical variables are also presented as frequency (percentage). Differences were assessed using chi-square test or Fisher’s exact test when needed. The significance level was set at 0.05 in two-tailed tests. Statistically significant variables in the underestimation and non-underestimation groups were included into the stepwise logistic regression analysis to identify factors associated with the underestimation of DCIS. All statistical analyses were performed in SPSS version 17 (released in 2008, SPSS Statistics for Windows, SPSS Inc., Chicago, IL, USA).
4. Results
A total of 288 patients with 296 DCIS lesions were identified based on the postoperative pathological examination. All patients were female, with a mean age of 53.8 ± 11.7 years. Eight patients were found to have two DCIS lesions each. Three of them had two lesions in the same breast, while five of them had two lesions in different breasts. Based on the pathology reports, underestimation occurred in 65 (22.0%) lesions. The rate of non-underestimation was 78.0% (231/296). However, there was no significant difference in terms of age and clinical presentations between the two groups of patients (Table 1).
| Underestimation (n = 65) | Non-underestimation (n = 231) | P-value | |
|---|---|---|---|
| Age (y, mean ± SD) | 50.3 ± 11.6 | 54.8 ± 11.8 | 0.010 |
| Clinical presentations, No. (%) | |||
| Breast pain | 9 (13.8) | 36 (15.6) | 0.732 |
| Palpable mass | 33 (50.8) | 122 (52.8) | 0.771 |
| Nipple discharge/bleeding | 13 (20.0) | 51 (22.1) | 0.486 |
Abbreviations: DCIS, ductal carcinoma in situ; BI-RADS, Breast Imaging-Reporting and Data System.
The ultrasound features of breast lesions with DCIS based on BI-RADS were compared between the underestimation and non-underestimation groups (Table 2). The underestimated lesions were more likely to have an oval shape (24.6% vs. 7.8%), a non-cumulus pattern (26.2% vs. 16.0%), a circumscribed margin (30.8% vs. 6.5%), and a non-parallel orientation (26.2% vs. 15.2%). Also, they were more likely to be hypoechogenic (44.6% vs. 27.7%), have no posterior acoustic changes (78.5% vs. 57.1%), and exhibit non-microcalcification (87.7% vs. 43.7%) with duct changes (20.0% vs. 4.8%); also, there was no blood flow in these lesions (40.0% vs. 12.6%). All differences between the two groups were statistically significant.
| Variables | Underestimation (n = 65) | Non-underestimation (n = 231) | P-value |
|---|---|---|---|
| Shape | < 0.001 | ||
| Oval | 16 (24.6) | 18 (7.8) | |
| Irregular | 49 (75.4) | 213 (92.2) | |
| Ultrasound pattern | 0.032 | ||
| Cumulus | 48 (73.8) | 194 (84.0) | |
| Coral | 10 (15.4) | 29 (12.6) | |
| Miscellaneous | 4 (6.2) | 7 (3.0) | |
| Pipe | 3 (4.6) | 1 (0.4) | |
| Margin | < 0.001 | ||
| Circumscribed | 20 (30.8) | 15 (6.5) | |
| Non-circumscribed | 45 (69.2) | 216 (93.5) | |
| Orientation | 0.039 | ||
| Parallel | 48 (73.8) | 196 (84.8) | |
| Non-parallel | 17 (26.2) | 35 (15.2) | |
| Echo pattern | < 0.001 | ||
| Anechoic | 1 (1.5) | 0 (0.0) | |
| Complex cystic and solid | 5 (7.7) | 9 (3.9) | |
| Heterogeneous | 26 (40.0) | 157 (68.0) | |
| Hyperechoic | 1 (1.5) | 0 (0.0) | |
| Hypoechoic | 29 (44.6) | 64 (27.7) | |
| Isoechoic | 3 (4.6) | 1 (0.4) | |
| Posterior features | 0.005 | ||
| No posterior features | 51 (78.5) | 132 (57.1) | |
| Enhancement | 5 (7.7) | 22 (9.5) | |
| Shadowing | 3 (4.6) | 7 (3.0) | |
| Combined pattern | 6 (9.2) | 70 (30.3) | |
| Architectural distortion | 0.214 | ||
| No | 65 (100.0) | 222 (96.1) | |
| Yes | 0 (0.0) | 9 (3.9) | |
| Microcalcifications | < 0.001 | ||
| No | 57 (87.7) | 101 (43.7) | |
| Yes | 8 (12.3) | 130 (56.3) | |
| Duct changes | < 0.001 | ||
| No | 52 (80.0) | 220 (95.2) | |
| Yes | 13 (20.0) | 11 (4.8) | |
| Vascularity | < 0.001 | ||
| Absent | 26 (40.0) | 29 (12.6) | |
| Internal vascularity | 39 (60.0) | 188 (81.4) | |
| Vessels in rim | 0 (0.0) | 14 (6.1) |
Abbreviations: DCIS, ductal carcinoma in situ; BI-RADS, Breast Imaging-Reporting and Data System.
a Values are expressed as No. (%).
In terms of pathological characteristics, DCIS cases in the underestimation group were more likely to have smaller lesions, with less frequent microinvasions and lower grade classifications (Table 3). Among breast lesions with a maximum diameter of ≤ 10 mm, 32.3% (21/65) were underestimated in the ultrasound BI-RADS assessment. When the maximum diameter of lesions was greater than 10 mm, no significant difference was found in the incidence of DCIS between the two groups. Further details are presented in Table 3.
| Variables | Underestimation (n = 65) | Non-underestimation (n = 231) | P-value |
|---|---|---|---|
| Maximum diameter | < 0.001 | ||
| ≤ 10 mm | 21 (32.3) | 14 (6.1) | |
| 10 - 30 mm | 39 (60.0) | 138 (59.7) | |
| 30 - 50 mm | 5 (7.7) | 61 (26.4) | |
| ≥ 50 mm | 0 (0.0) | 18 (7.8) | |
| Microinvasion | < 0.001 | ||
| No | 45 (69.2) | 85 (36.8) | |
| Yes | 20 (30.8) | 146 (63.2) | |
| Van Nuys classification | < 0.001 | ||
| Grade 1 | 31 (47.7) | 50 (21.7) | |
| Grade 2 | 23 (35.4) | 138 (59.7) | |
| Grade 3 | 11 (16.9) | 43 (18.6) |
Abbreviations: DCIS, ductal carcinoma in situ; BI-RADS, Breast Imaging-Reporting and Data System.
a Values are expressed as No. (%).
The demographic characteristics, clinical manifestations, pathological characteristics, and age of the patients, besides the diameter, microinvasion, shape, margin, orientation, echo pattern, posterior acoustic features, ultrasound pattern, and vascularity of the lesions, were included in a stepwise logistic regression analysis. However, only age, maximum diameter, absence of microinvasion, and margin were identified as factors that may lead to the underestimation of DCIS in the BI-RADS assessment. The odds ratio (OR) and 95% confidence interval (95% CI) of the mentioned factors are presented in Table 4.
| Variables | Level of variable | OR (95% CI) | P-value |
|---|---|---|---|
| Age (y) | ≥ 50 | 2.839 (1.449 - 5.560) | 0.002 |
| Diameter (mm) | ≤ 10 | 16.454 (4.328 - 62.560) | < 0.001 |
| Microinvasion | Yes | 3.308 (1.701 - 6.436) | < 0.001 |
| Margin | Circumscribed | 6.536 (2.746 - 15.555) | < 0.001 |
Abbreviations: DCIS, ductal carcinoma in situ; BI-RADS, Breast Imaging-Reporting and Data System.
5. Discussion
Ultrasound is a widely used imaging modality in the clinical setting. Its diagnostic performance depends on the size, location, and characteristics of the lesion, as well as the operator's experience (23, 24). Small lesions with a heterogeneous echo pattern and unclear boundaries can easily lead to a difficult diagnosis via ultrasound (25). In the present study, the sensitivity of ultrasound BI-RADS classification to assign a breast DCIS lesion into a higher (≥ 4B) grade lesion was 78.0%, which could vary in different clinical settings. The present results showed no significant differences in the age and clinical presentations of the patients between the underestimation and non-underestimation groups, based on the ultrasound BI-RADS classification. This finding is consistent with previous reports, which suggested that the diagnosis of breast cancer could not completely rely on clinical features (26, 27).
Based on the pathology reports, breast lesions with underestimation of DCIS were more likely to be smaller, with less frequent microinvasions and lower grade classifications. When the maximum diameter of the breast lesion was ≤ 10 mm, about one-third of lesions were underestimated. It was difficult to detect small lesions by ultrasound (28). Small lesions do not commonly have the typical signs of malignant lesions, such as irregular borders, microcalcifications, and abundant blood flow signals (29). Compared to DCIS with microinvasions, DCIS without microinvasions was more likely to be characterized by hypoechogenicity, smooth borders, and less blood flow (30). Breast lesions classified as Van Nuys grade 1 were less likely to have microcalcifications and abundant blood flow signals, which could lead to a missed diagnosis via ultrasound and underestimation of the BI-RADS assessment (30). According to the present results, in the clinical setting, physicians should be cautious when evaluating a breast lesion with a maximum diameter of ≤ 10 mm. Other ultrasound features should be also considered to reduce the missed diagnosis of potential malignant lesions.
Typical ultrasound features for DCIS include dilated ducts, thickened duct walls, a cumulus or coral pattern, a hypoechoic echo pattern, intraductal needle-like or granular microcalcifications, and abundant intraductal blood flow signals (31, 32). Based on the comparison of ultrasound features between patients with underestimation and non-underestimation of DCIS according to the BI-RADS assessment, significant differences were found in the lesion shape, ultrasound pattern, margin, orientation, echo pattern, posterior acoustic features, microcalcifications, duct changes, and vascularity of lesions.
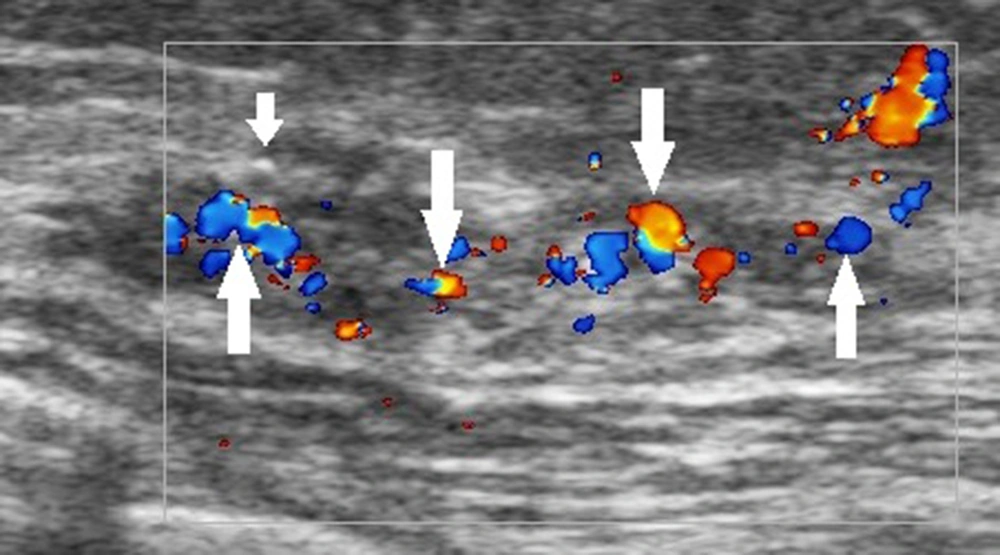
The ultrasound BI-RADS lexicon simply divides the blood flow in the breast lesion into three types: absence of blood flow, internal vascularity, and vessels in rim. Nevertheless, both benign and malignant breast lesions may have any of these features. For example, intraductal papilloma, atypical fibroadenoma, and intramammary lymph nodes may all exhibit abundant internal vascularity. With advances in color Doppler ultrasound, more sonographic blood flow features can be identified (33). Clinically, the presence of abundant blood flow signals within the thickening ducts of breast lesions can be used as an important sonographic feature to distinguish DCIS from inflammatory breast lesions, such as plasma cell mastitis and granulomatous mastitis (Figure 1); both lesions can have a rich blood flow in the extraductal stroma. Besides, hypoechogenicity without blood flow signals is an important ultrasound feature that indicates a cystic lesion or intraductal lipid deposition rather than DCIS. A complex breast lesion with detectable blood flow signals requires further studies. These new features based on color Doppler ultrasound need to be considered in the BI-RADS classification of breast lesions.
A 42-year-old patient with a definite diagnosis of ductal carcinoma in situ (DCIS). The ultrasound examination shows an occupying lesion in the superior lateral quadrant of the left breast, with multiple microcalcifications (short arrow) and abundant blood flow (long arrows) in the enlarged ducts. The Breast Imaging-Reporting and Data System (BI-RADS) classification indicated a 4C category, and the pathology report indicated a medium-to-high grade DCIS.
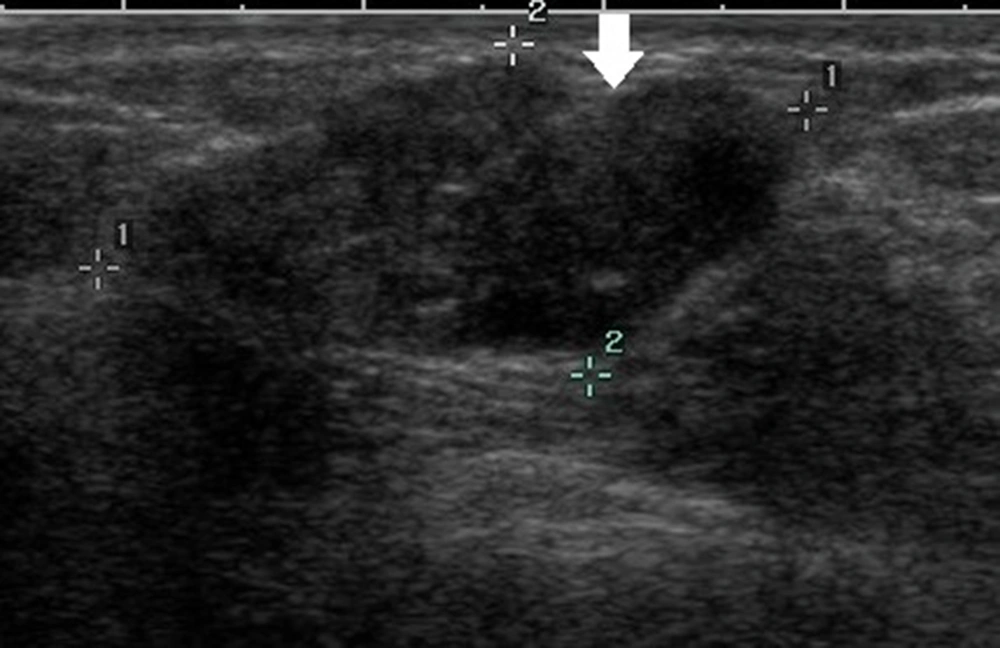
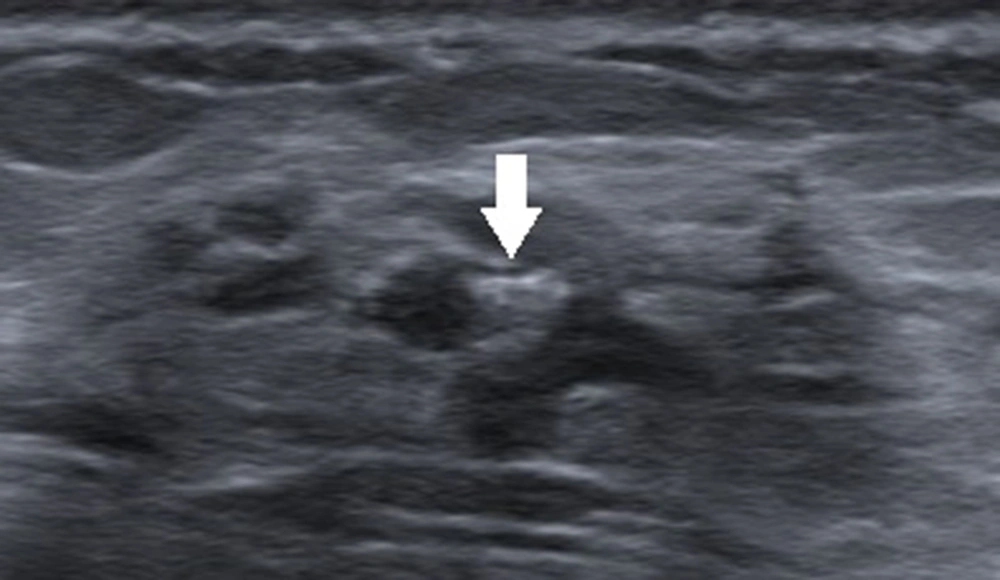
Generally, an oval-shaped breast lesion is considered a benign lesion (34). However, in the present study, in breast lesions with underestimation of DCIS, 24.6% of lesions (16/65) had an oval shape, whereas only 7.8% (18/231) were oval in the non-underestimation group. Among 16 oval-shaped breast lesions in the underestimation group, 10 breast lesions had a maximum diameter of ≤ 10 mm. Four patients had intraductal papilloma with carcinoma in situ. One patient had a cystic lesion with no blood flow signals in the solid segment, and one patient had a lobulated oval lesion. For lesions with large lobes, visual assessment may be difficult, as it is challenging to distinguish them from an irregular-shaped lesion (Figure 2). Physicians should pay close attention to small breast lesions or lesions with large lobes, as they are usually oval or have an irregular shape, which can easily lead to misclassification.
A 47-year-old patient with a misdiagnosis of ductal carcinoma in situ (DCIS). The ultrasound examination shows an occupying lesion in the inferior inner quadrant of the left breast with a parallel orientation, a large, lobulated, and oval shape (arrow), and circumscribed margins. Strong echogenic heterogeneous distributions are also detected. The Breast Imaging-Reporting and Data System (BI-RADS) classification indicated a BI-RADS 4A lesion, and the pathology report indicated high-grade DCIS with focal microinvasions.
In this regard, a previous study showed that the most common reason to miss a malignant breast lesion was failure to recognize its suspicious margins (35). There were 35 patients with circumscribed margins in the present study. Twenty cases (57.1%) were underestimated in the BI-RADS assessment. Fifteen patients had intraductal or cystic lesions. These lesions could be better classified into solid, cystic, and complex types based on color Doppler ultrasound. Cystic or complex breast lesions may have a 22.8 - 25.0% chance of malignancy (36, 37), corresponding to a higher category in the BI-RADS classification; this finding again suggests the importance of incorporating the results of color Doppler ultrasound into the BI-RADS classification.
The majority of DCIS lesions identified in this study had a heterogeneous (61.8%, 183/296) or hypoechoic (31.4%, 93/296) echo pattern. DCIS lesions with a hypoechoic echo pattern (31.2%, 29/93) were more likely to be underestimated compared to those with a heterogeneous echo pattern (14.2%, 26/183). Since many benign lesions, such as breast fibroadenoma, often have a hypoechoic pattern, echo pattern alone has limited value when distinguishing between benign and malignant lesions. In previous studies, 7.7 - 15.0% of lesions with duct changes were malignant (38-42). In the present study, 13 patients (20.0%) with duct changes were underestimated. Eleven patients were considered to have intraductal papilloma and were classified into the BI-RADS 4A category. The final pathology reports indicated intraductal papilloma with carcinoma in situ. It is generally difficult to distinguish between benign and malignant breast intraductal lesions. Lesions with the following signs may be associated with malignancy: lesions located in the surrounding duct (> 2 cm from the nipple); single rather than multiple ductal dilations; a thickened duct wall; segmented rather than focal intraductal contents; abundant blood flow signals in the intraductal content; and nipple bleeding.
Calcifications in the breast can be classified as macrocalcifications or microcalcifications, with the former commonly seen in benign lesions and the latter more frequently observed in malignancies. Ultrasound can detect microcalcifications within the breast lesion, but with lower sensitivity compared to mammography, especially for microcalcifications with a diffuse distribution in the lesion (43, 44). DCIS with microcalcifications may have typical ultrasound features of fine strong echogenic dots along the thick hypoechogenic duct wall. In this study, eight lesions with microcalcifications were underestimated, and five lesions were small (maximum diameter, 5 - 9 mm). Two lesions were underestimated due to the accumulation of microcalcifications, which resembled macrocalcifications. The last lesion showed a diffuse distribution of microcalcifications in the entire upper half of the breast.
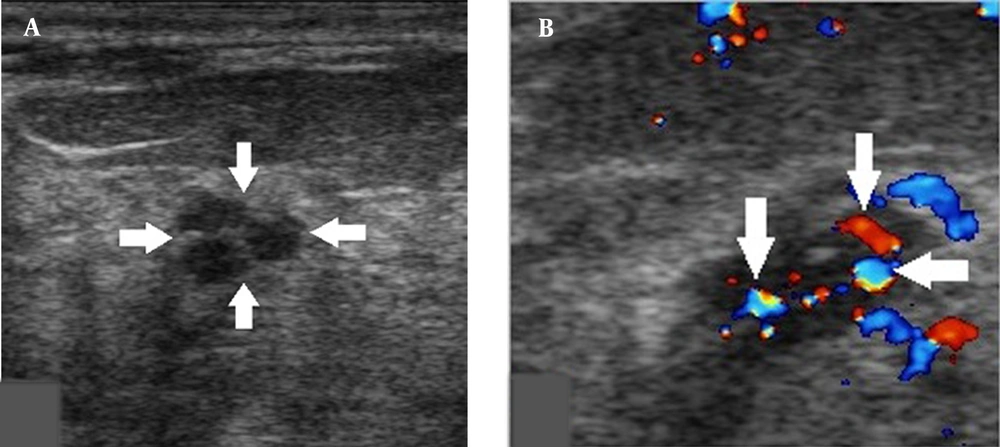
Small breast DCIS lesions can only show 1 - 2 microcalcifications and do not exhibit typical ultrasound features of tubular or cluster distributions of microcalcifications, which can easily lead to underestimations. Classification of breast lesions, together with the consideration of intralesional blood flow signals, may improve the accuracy of classification (Figure 3). Physicians should be also careful about macrocalcifications with rough surfaces, as they might be caused by the accumulation of microcalcifications (Figure 4). Evaluation of calcifications can be also affected when there are poor ultrasound penetrations from diffuse dilated mammary ducts. Further examination via mammography can help characterize calcifications (Figure 5).
A, A 44-year-old patient with a misdiagnosis of ductal carcinoma in situ (DCIS). The ultrasound examination shows a hypoechoic mass in the superior lateral quadrant of the right breast, with an irregular shape and circumscribed margins in a parallel orientation. Strong echogenic foci were found without acoustic shadowing and no changes posterior to the mass (arrows). B, Color Doppler flow imaging shows abundant blood flow signals in the mass (arrows). The Breast Imaging-Reporting and Data System (BI-RADS) classification indicated a category 3 lesion, and the pathology report indicated a low-grade DCIS.
A 46-year-old patient with a misdiagnosis of ductal carcinoma in situ (DCIS). The ultrasound examination shows a hypoechoic mass in the superior lateral quadrant of the right breast, with an irregular shape and coarse calcification (arrow). The Breast Imaging-Reporting and Data System (BI-RADS) classification indicated a category 3 lesion, and the pathology report indicated a low-grade DCIS lesion with microinvasions.
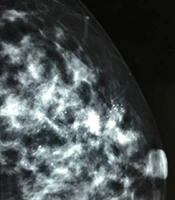
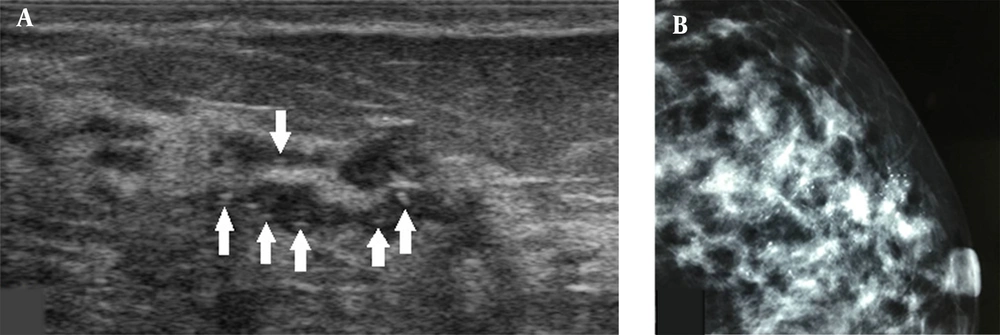
A, A 34-year-old patient with a misdiagnosis of ductal carcinoma in situ (DCIS). The ultrasound examination shows ductal dilations in the superior lateral quadrant of the left breast with poor ultrasound penetration and a weak echo pattern. There are scattered strong echogenic foci without acoustic shadowing (arrows), but with focal blood flow signals. The Breast Imaging-Reporting and Data System (BI-RADS) classification indicated a category 4A lesion, and the pathology report indicated intermediate-grade DCIS with microinvasions. B, Microcalcifications are found in the mammogram. The lesion was classified as BI-RADS 5.
It is suggested to classify a breast lesion into the BI-RADS 0 category if it has localized extraductal diffuse microcalcifications. A breast lesion with bilateral diffuse microcalcifications can be categorized into BI-RADS 2, but can be also classified into BI-RADS 0, based on further examinations by mammography, as a mammogram can better determine the characteristics of microcalcifications. In terms of the ultrasound pattern, underestimated rates were higher in lesions with miscellaneous and pipe patterns compared to those with cumulus and coral patterns. Physicians need to familiarize themselves with these patterns to improve the accuracy of BI-RADS classification for DCIS.
The limitations of this study include its single-center scope, small sample size, and retrospective design. Ultrasound results can also vary depending on the clinical setting, menstruation period, and the experience of ultrasonographers. Also, mammography was not performed for every patient, making it difficult to correlate the ultrasound findings with mammography results.
In conclusion, 22% of DCIS lesions were underestimated based on the ultrasound BI-RADS assessment in this study. In DCIS lesions, underestimations were more frequently observed in the breast lesions of patients aged above 50 years, with a maximum lesion diameter of < 10 mm, no microinvasion, and circumscribed margins.